How to Comply with Medicare Signature & Documentation Requirements
Failure to comply with Medicare signature and authentication rules is a frequent cause of claims denials. In January, the Centers for Medicare & Medicaid Services (CMS) revised its Medicare signature guidelines to incorporate new rules for electronic signatures. Here’s an overview of the changes along with a general overview of what lab compliance managers need to know about Medicare signature rules to minimize risk of claims denials. Medicare Signature Rules Medicare rules require the ordering or prescribing physician or Non-Physician Practitioner (NPP) to make a mark or sign on a document indicating his/her knowledge, approval, acceptance, or obligation to services provided or certified. The purpose of the rule is to demonstrate that services billed to Medicare have been accurately and fully documented, reviewed, and authenticated. The Medicare Claims Review Contractor reviews documentation to ensure it meets signature requirements and may deny claims if it doesn’t. The rules require that the signature for each entry be legible and include the practitioner’s first and last name. If the signature isn’t legible, the reviewer must confirm it by comparing it to a log or attestation statement. CMS recommends that practitioners also list their credentials, e.g., M.D., P.A., or D.O., for purposes of clarification. […]

Medicare Signature Rules
Medicare rules require the ordering or prescribing physician or Non-Physician Practitioner (NPP) to make a mark or sign on a document indicating his/her knowledge, approval, acceptance, or obligation to services provided or certified. The purpose of the rule is to demonstrate that services billed to Medicare have been accurately and fully documented, reviewed, and authenticated. The Medicare Claims Review Contractor reviews documentation to ensure it meets signature requirements and may deny claims if it doesn’t. The rules require that the signature for each entry be legible and include the practitioner’s first and last name. If the signature isn’t legible, the reviewer must confirm it by comparing it to a log or attestation statement. CMS recommends that practitioners also list their credentials, e.g., M.D., P.A., or D.O., for purposes of clarification.Signature Logs & Attestations
The New Electronic Signatures Guidance
Acceptable signatures include handwritten signatures or initials, as well as electronic signatures. The latter typically include date and timestamps and printed statements, “electronically signed by” or “verified/reviewed by,” followed by the practitioner’s name and a professional designation. Also acceptable are digital signatures, or written signature typically generated by encrypted software, allowing sole usage. The revised CMS guidance clarifies that electronic signatures, including an electronic sound, symbol, or process attached to, or logically associated with an electronic medical record, must be:- Authenticated;
- Safeguarded against misuse and modification; and
- Easily identifiable as electronic instead of a typed signature.
Non-Acceptable Signatures
Medicare doesn’t accept rubber stamped signatures. Exception: A rubber stamp is okay if the practitioner has a physical disability and can prove to a CMS contractor that he/she is unable to sign due to that disability. Nor does it accept scribe signatures, even if the scribe dictates the entry on the practitioner’s behalf.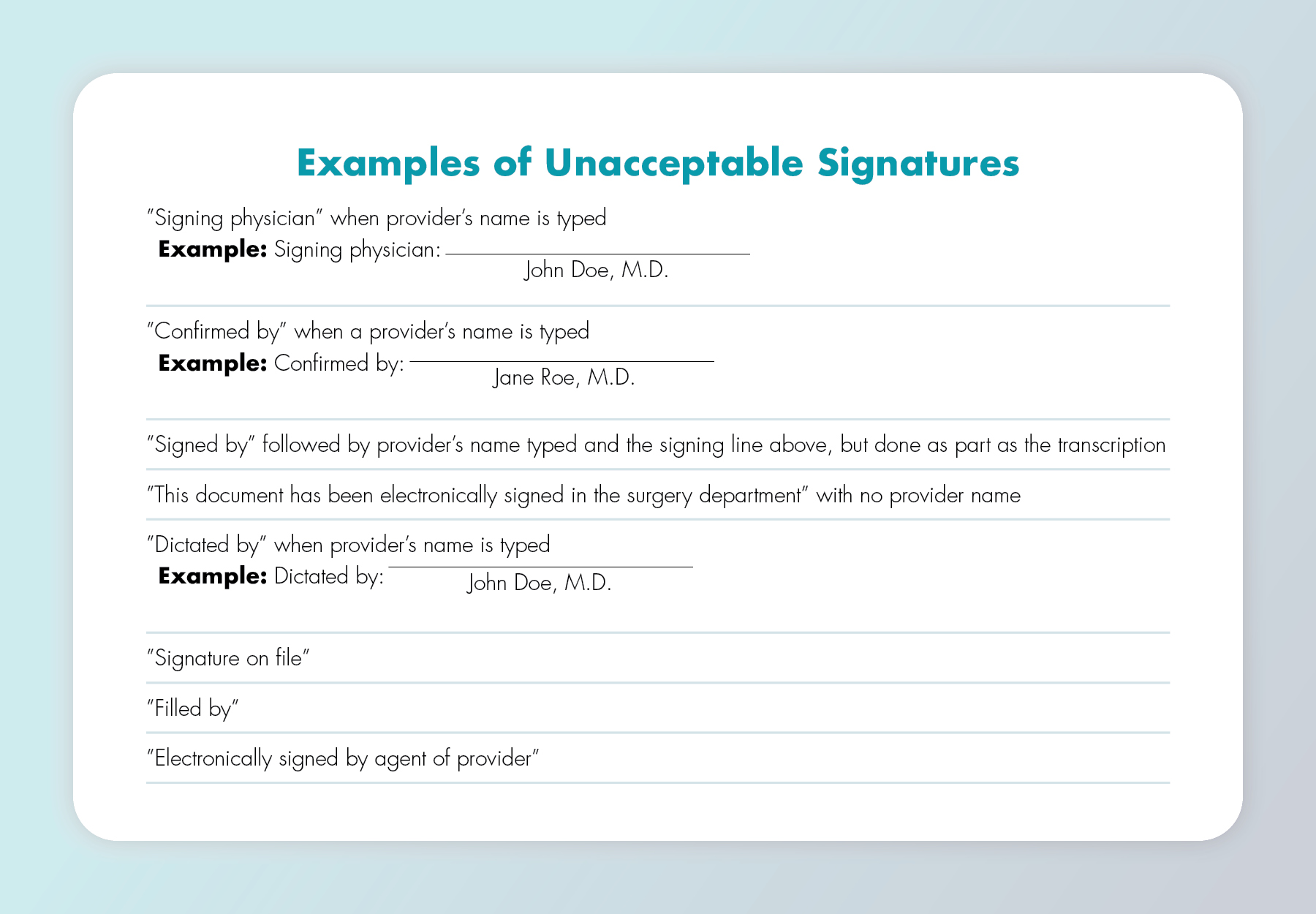
Medicare Medical Documentation & Order Requirements
In addition to a signature or marking, Medicare rules require that billed services include proper medical documentation confirming that the physician or NPP ordered and verified the medical necessity and reasonableness of the tests or other services billed. Such documentation includes orders indicating a patient’s need for the tests, as well as lab results and clinical observations. The documentation must have enough information to show the date the tests were ordered or performed. Medicare will deny the claim if the submitted medical record doesn’t include an order. Orders must be signed but exceptions apply to certain tests. When and if such an exception applies and the order is unsigned, there must be medical documentation, such as a progress note from the treating physician indicating that he/she intended that the test be performed. Documentation of intent must be authenticated by the author via a handwritten or electronic signature. Missing orders may not be created after the fact to backdate a plan of care or other service, and you can’t add late signatures to orders or medical records (beyond the short delay that happens during the transcription process). Retroactive orders are unacceptable, with certain exceptions, e.g., an unsigned clinical diagnostic order may be accepted if there’s a signed progress note in the record indicating the practitioner’s intent to order the test. If you date the entries immediately above and below an undated entry, medical review may reasonably assume the entry date in question.Other Unsigned Medical Records
If other medical records aren’t signed, you must send an attestation statement. While rules vary slightly by Medicare administrative contractor, the general rule is that upon the request of a claim reviewer, you must submit an attestation statement from the physician or NPP or signature log to authenticate a medical record within 20 calendar days. Attestations won’t work for unsigned orders, though.Subscribe to view Essential
Start a Free Trial for immediate access to this article