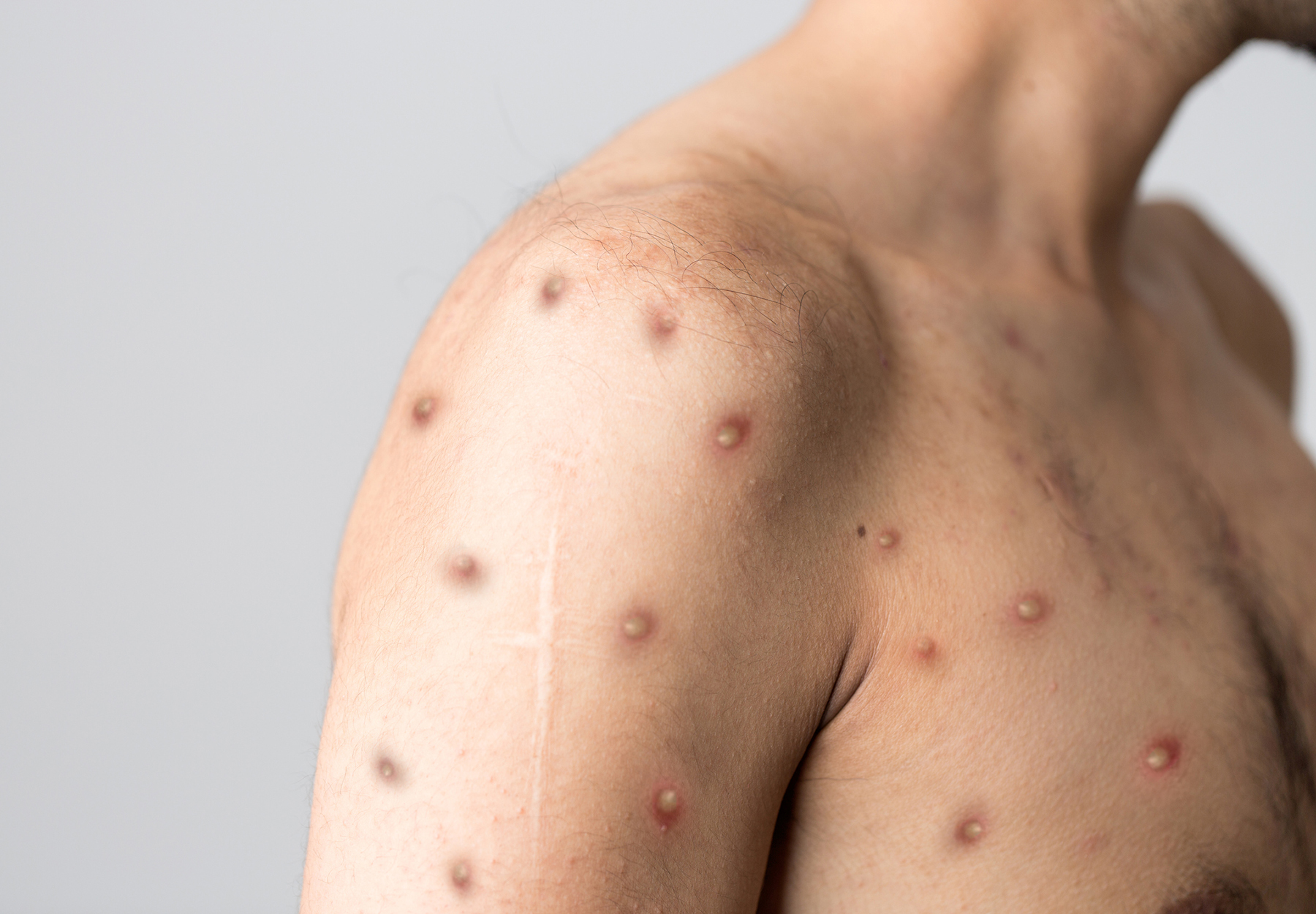
When it comes to testing for potential cases of monkeypox, swab samples taken directly from a patient’s lesion are the best option to avoid false results, according to a July 15 Safety Communication from the FDA. The agency stated that other sample types, such as saliva or blood, may result in either false negatives or false positives for monkeypox and that it’s not aware of clinical data supporting the use of these sample types.
Both false negatives and false positives could lead to patients not receiving the correct diagnosis and treatment for the condition they do have, as well as cause further spread of the monkeypox virus in the case of someone receiving a false negative and not isolating, the FDA explains.
The agency urges test developers to reach out regarding alternate testing approaches and new test development, as well as to share validation data from other sample types such as blood or saliva, via MPXDx@fda.hhs.gov.
The World Health Organization (WHO) also recommends skin lesions as the ideal sample type for diagnosing monkeypox via PCR testing, which it calls “the preferred laboratory test[,] given its accuracy and sensitivity.” According to WHO, skin lesions include the fluid or roof from pustules and vesicles, and dry crusts. Such samples should be stored in a sterile, dry tube with no viral transport media and kept cold, WHO adds.
In terms of other sample types, WHO says that “PCR blood tests are usually inconclusive because of the short duration of viremia [presence of viruses in the blood] relative to the timing of specimen collection after symptoms begin and should not be routinely collected from patients.”
Some of the current tests for diagnosing monkeypox include:
- The FDA-cleared US Centers for Disease Control and Prevention’s (CDC’s) RT-PCR test, which uses a lesion sample to detect monkeypox
- The Bioperfectus Monkeypox Virus Real Time PCR Kit, a CE-marked kit that detects the virus on either a human tonsillar swab, nasopharyngeal swab, serum, whole blood, lesion exudate, or scab
- Anitoa Systems’ CE-marked PCR solution for monkeypox testing, which detects the virus in skin lesion samples
There are also some research use only tests available on the market from manufacturers such as Roche, Gold Standard Diagnostics (a Eurofins company), and Novacyt, as well as several other test options currently in development by companies including Co-Diagnostics, Becton Dickinson, CerTest Biotec, Cepheid, and BioGX.
The current outbreak includes several countries that do not have a history of monkeypox, with a total of 13,340 cases in 69 locations around the world reported as of July 19, according to the CDC. Of those cases, 13,100 were from 63 locations that have not historically reported monkeypox.