Doing More with Less
A new capillary blood collection system, the BD MiniDraw™, can improve the testing experience for both labs and patients
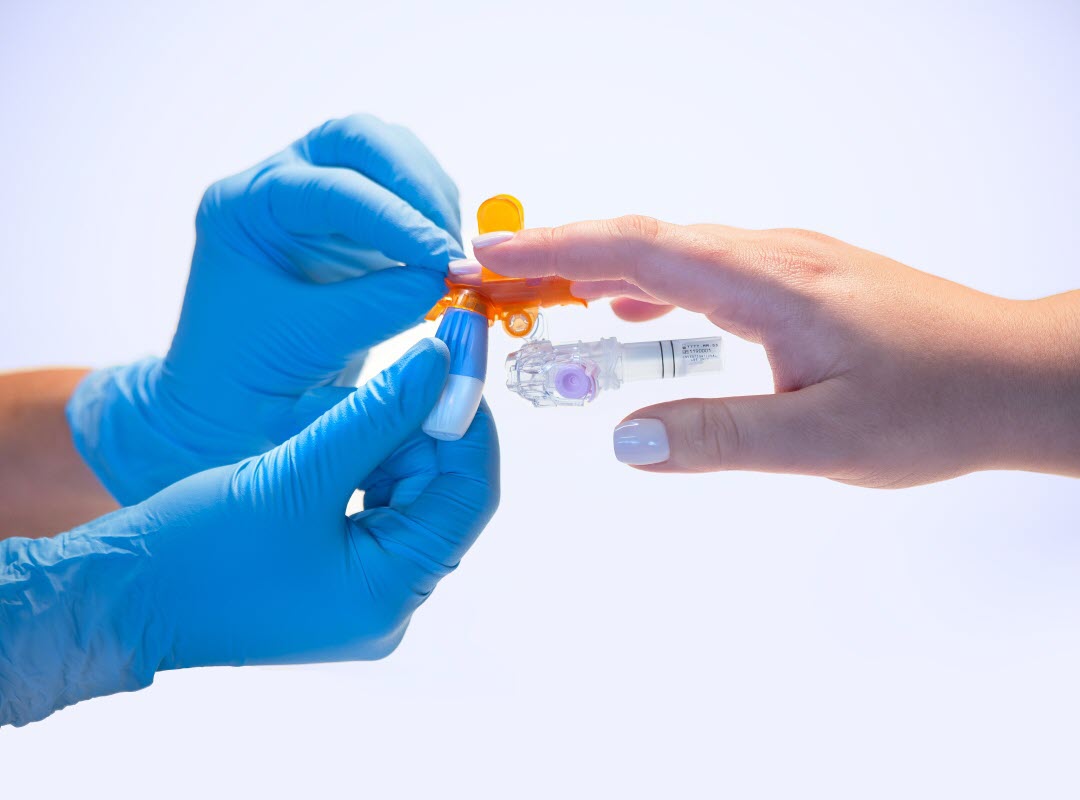
The BD MiniDraw™ capillary blood collection system, which received FDA 510(k) clearance in December 2023, is a fingertip draw system that requires very low volumes of blood to deliver high-quality test results.1 The device is intended for use by healthcare professionals without specific experience in phlebotomy, creating opportunities for greater convenience and portability in patient care. But what sets it apart from other capillary sampling devices—and what advantages does it offer for the clinical laboratory?
Better samples, better care
“Compared with other fingerstick-based collection devices, there are a number of benefits,” explains Russell Heinrich, vice president of research and development in specimen management at BD. “One is that it has some automated features, so it’s very standardized; someone without a lot of phlebotomy experience can get high-quality results.” Not only does this increase patients’ access to care, but it also significantly reduces the degree of hemolysis.2 “We are able to get high-quality results, especially in analytes that are very sensitive to hemolysis. That also means less work for the lab—fewer retests and a lower likelihood of error are big benefits.”
Another advantage for labs is that each tube comes pre-barcoded, further simplifying sample accessioning and processing and reducing the risk of error. Although the system requires some advance preparation, Heinrich believes the outcome is worth the effort. “When labs are set up for it, a lower volume of blood requires lower volumes of reagents. There’s less blood and reagent to discard, there’s less waste, and there’s more efficiency overall.”
It’s not only laboratorians who benefit from the system. “From a patient point of view, it’s more comfortable. Having your finger milked is quite different to having a device attached to your finger that performs some gentle squeezing over a minute or two,” says Heinrich. “From a venous collection point of view, as many as one in four people have a strong fear of needles. This device doesn’t look like a needle—and you don’t see the needle—so it seems much less scary. We did a patient preference study that showed that 65 percent of patients preferred this over traditional approaches.3 That’s a big difference.” Many people also need or prefer lower blood collection volumes, which can increase patient comfort and decrease risk of anemia or blood loss.4
Capillary collection: who and how?
The system can be used across a variety of patient types, from healthy people undergoing routine checkups to those who require frequent monitoring for chronic diseases. “A common use case we have encountered is the patient who goes to their primary care physician and needs a blood test as a follow-up,” says Heinrich. “Rather than having to schedule something at a blood collection center, they just go to the pharmacy to pick up their prescription—and while they’re there, they can pick up a quart of milk and get their blood drawn at the same time.” Doctors’ offices are another target for the technology; enabling healthcare professionals to perform the collections patients need on-site can speed up the time to diagnosis and treatment, increase adherence to testing and follow-up care, and enhance the overall comfort and convenience of the patient experience.
Many patients who receive diagnostic test referrals never complete the testing5—sometimes due to a needle phobia,6 sometimes because paperwork is lost or appointments forgotten,7 and sometimes simply because the process of booking appointments, traveling to a collection center, and undergoing venipuncture requires time and effort.8 Capillary sampling can help mitigate these concerns by removing the need for travel and offering a quick, convenient procedure that can take place at the point of care or alongside other routine tasks such as grocery shopping.
“We believe this will also have applicability for patients who have difficult venous access,” adds Heinrich. “Some patients, especially elderly people or those undergoing certain treatments, have veins that are very hard to access or collapse easily. Can a capillary collection system help them, too? We think so.”
Currently, the BD MiniDraw™ system is cleared for 16 analytes including lipid panels, clinical chemistry, and hemoglobin and hematocrit testing. Heinrich anticipates that the test menu will expand to include other tests commonly ordered in primary care settings or used to monitor chronic diseases. He also anticipates moving into new testing locations; although the initial launch will be in retail pharmacies, he says, “We don’t think we should stop there. The consumer view of healthcare has changed a lot, especially since COVID-19. I think customers’ expectations are going to be completely different—so doctors’ offices and at-home testing are areas of interest. We’re hugely driven by our mission of blood testing and what it can do for the world, so we’re always looking for new ways to impact that.”
Expanding horizons for blood testing
What’s the next step for the system? “Testing and prevention are much more effective than downstream treatment, so we want to advocate for that in every patient population,” says Heinrich. “We’re always searching for the next big disease, analyte, or opportunity. When we announced that the BD MiniDraw™ had received clearance, a lot of people reached out to ask whether and how they could use it in their testing. We’re now vetting those queries to figure out which ones make the most sense, but they are all opportunities that will put blood collection at our patients’ fingertips.”
Although capillary blood collection may not be the answer for every patient, Heinrich and his colleagues are optimistic that it will open up more opportunities to provide rapid, convenient, and comfortable access to laboratory testing. The BD MiniDraw™ system is expected to make its debut in the latter half of 2024 at retail pharmacies in Texas. “We’re excited to see something we’ve developed impact patients in a positive way—so we’re looking forward to having the first external blood draw and seeing where things go next,” says Heinrich. “To us, it’s just the first step in a longer journey to transform healthcare.”
References:
- BD. BD Receives FDA 510(k) Clearance for Potentially Transformative Fingertip Blood Collection Device. December 7, 2023. https://news.bd.com/2023-12-07-BD-Receives-FDA-510-k-Clearance-for-Potentially-Transformative-Fingertip-Blood-Collection-Device.
- Parikh M, Berube J. Reduced hemolysis with a novel capillary collection system as compared with conventional capillary collection devices. Clin Chem. 2023;69:hvad097.160. doi:10.1093/clinchem/hvad097.160.
- Pourafshar S et al. An assessment of individual preference for a novel capillary blood collection system. Patient Prefer Adherence. 2024;18:531–541. doi:10.2147/PPA.S437969.
- Spethmann J et al. Laboratory medicine contributions to patient blood management concepts. J Lab Med. 2018;42(3):81–87. doi:10.1515/labmed-2017-0148.
- Ramsay N et al. Investigating patient adherence with pathology testing in primary care and how point of care testing can improve it. Point Care. 2016;15(4):144–151. doi:10.1097/POC.0000000000000110.
- Alsbrooks K, Hoerauf K. Prevalence, causes, impacts, and management of needle phobia: An international survey of a general adult population. PLoS One. 2022;17(11):e0276814. doi:10.1371/journal.pone.0276814.
- Moffett HH et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339–344.
- Toxopeus DCM et al. Compliance with laboratory monitoring guidelines in outpatient HIV care: a qualitative study in the Netherlands. AIDS Care. 2019;31(7):840–847. doi:10.1080/09540121.2018.1563280.
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article