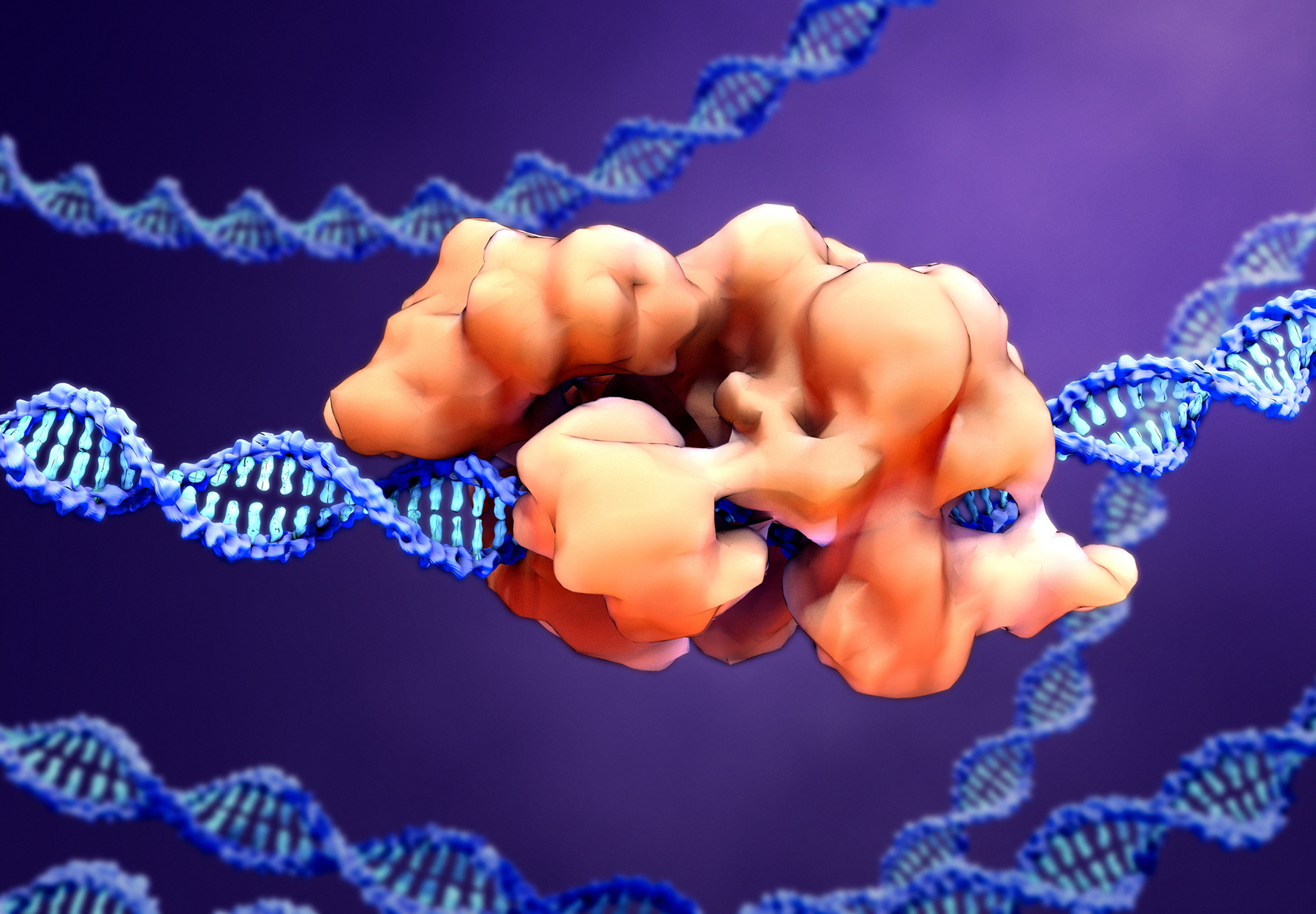
A significant progression in COVID-19 test development occurred on Jan. 24, when the US Food and Drug Administration (FDA) announced that it granted Emergency Use Authorization (EUA) for a high-throughput assay that uses CRISPR technology to detect the SARS-CoV-2 virus. The CRISPR-based assay provides PCR-equivalent performance but on a much more scalable and rapid basis, potentially making it ideal for use in screening asymptomatic populations.
The Mammoth Biosciences DETECTR Kit
The DETECTR Boost SARS-CoV-2 Reagent Kit assay performs simultaneous reverse transcription and isothermal amplification using loop-mediated amplification (RT-LAMP) for RNA extracted from oral or nasal swabs, with a total run time of 15 minutes. The test was developed by Mammoth Biosciences, a San Francisco-based biotech company founded in 2017 by CRISPR pioneer Jennifer Doudna and colleagues from the University of California, Berkeley.
The DETECTR test is the product of a year-long collaboration between Mammoth and Agilent. The strategy: Tailor Mammoth’s CRISPR enzymes for identifying genetic codes to react with and identify the SARS-CoV-2 virus genome while Agilent leverages its Bravo BenchCel DB workstation platform to deliver high-speed liquid handling hardware to develop a test that can process more than 4,000 samples per day.
Financing & Marketing
Mammoth raised $195 million in Series C and D financing rounds in September 2021. The project has also received federal funding through the National Institutes of Health’s Rapid Acceleration of Diagnostics Initiative, as well as the Department of Health and Human Services.
Mammoth and Agilent will launch the test under a co-marketing agreement signed last January “This partnership will help address the need for more widespread testing options for COVID-19, helping to fill the gap in the market as testing labs run into supply issues or reach capacity,” noted Mammoth CEO Trevor Martin in
a statement issued when the co-marketing deal with Agilent was announced.
Here are some of the other important new FDA EUAs and clearances that were announced starting in late December 2021:
New FDA Emergency Use Authorizations (EUAs) & Approvals
| Manufacturer(s)
|
Product
|
| Maxim Biomedical
|
EUA for MaximBio ClearDetect COVID-19 Antigen Home Test for over-the-counter use |
| Mammoth Biosciences
|
EUA for DETECTR Boost SARS-CoV-2 Reagent Kit, a high-throughput SARS-CoV-2 assay utilizing CRISPR technology with PCR-equivalent performance
|
| Diadem
|
Breakthrough device designation for AlzoSure Predict blood-based biomarker prognostic assay for early prediction of Alzheimer's disease
|
| iHealth
|
EUA for iHealth COVID-19 Antigen Rapid Test Pro
|
| Pathogenomix
|
Breakthrough device designation for Patho-Seq assay
|
| Rheonix
|
510(k) approval for Rheonix STI TriPlex Assay for detection and differentiation of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis
|
| 23andMe
|
510(k) approval for direct-to-consumer genetic hereditary prostate cancer genetic risk report based on the HOXB13 marker |
| Premier Medical Laboratory Services
|
EUA for PMLS PCR-based SARS-CoV-2 assay |
| Becton Dickinson |
510(k) approval for BD Kiestra IdentifA system for automating sample preparation component of microbiology bacterial identification
|
| Qiagen |
Expanded approval of QuantiFeron-TB Gold assay for latent TB detection for use on individuals with weakened immune systems, pregnant women, and children
|
| Amazon
|
Expanded EUA for Amazon Real-Time RT-PCR Test for Detecting SARS-CoV-2 for use onsite at Amazon facilities or at home via unsupervised use of the test-collection kit
|
| Copan Diagnostics |
510(k) approval for Colibri automated clinical microbiology system
|
| Meridian Bioscience
|
Approval for Curian Campy rapid immunoassay for detection of Campylobacter infection
|
| Werfen |
510(k) approval for GEM Hemochron 100 whole blood hemostasis system |
| Siemens Healthineers |
EUA for over-the-counter Clinitest Rapid COVID-19 Antigen Self-Test |
| Roche
|
EUA for rapid, over-the-counter COVID-19 test
|
New CE Marks & Global Certifications
Notable European CE certifications announced during the period:
NEW CE MARKINGS IN EUROPE
| Manufacturer(s) |
Product(s) |
| Deep Sensing Algorithms |
BreathPass, first handheld breath test system for rapid detection of COVID-19 to get CE marking |
| Canary Global |
DigiGene COVID-19 Rapid Molecular Test Kit |
| United PPE America |
CRISPR SARS-CoV-2 test kit using genetic engineering technology licensed from Sherlock Biosciences |
| Inivata |
RaDaR assay to detect residual disease in cancer patients |
| LumiraDx |
LumiraDx CRP portable, microfluidic immunoassay for quantitative determination of C-reactive protein in direct fingerstick, venous whole blood, and plasma samples |
| Indica Labs |
HALO AP platform for anatomic pathology labs |
Other international clearances announced during the period:
| Manufacturer(s)
|
Country(ies) |
Product(s)
|
| Seegene |
Canada
|
Allplex SARS-CoV-2/FluA/FluB/RSV Assay
|
| Qiagen + Ellume |
Global Fund's Expert Review Panel Diagnostics
|
Battery-powered QiaReach QuantiFeron-TB test for use in low-resource, high-burden countries |
| CoSara Diagnostics
|
India
|
Saragene Human Papillomavirus High-Risk Real-Time PCR test |