Arrayit President Convicted of Silicon Valley Blood Testing Scam
Mark Schena was convicted of swindling investors and running a $77 million kickback scheme involving COVID-19 and allergy tests.
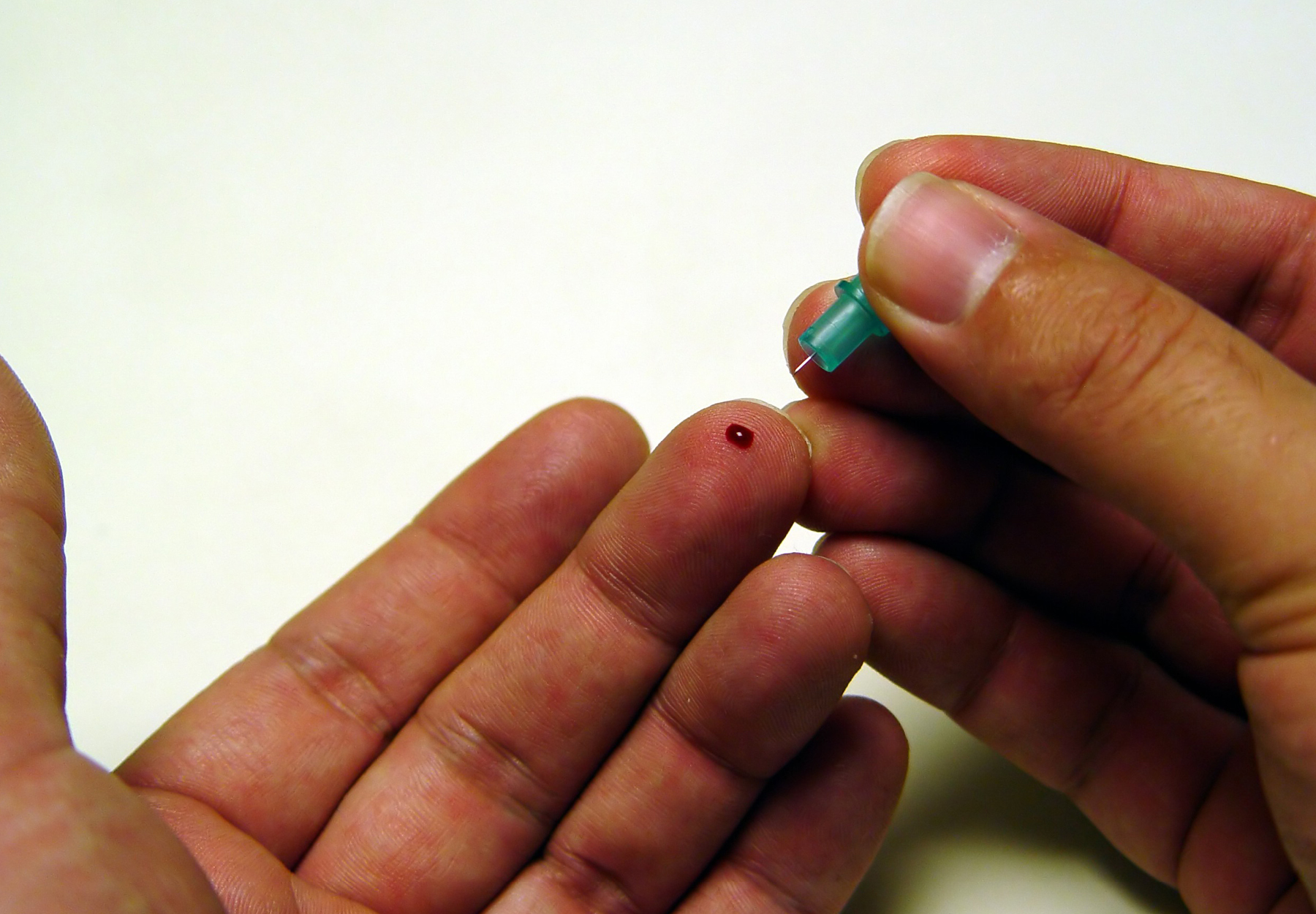
A federal jury in California has convicted the president of a Silicon Valley medical technology firm for fraudulent boasts about the company’s revolutionary technology capable of diagnosing just about any disease on the planet from just a few drops of blood. No, we’re not talking about Elizabeth Holmes, Sunny Balwani, or even Theranos. This familiar story features a new cast of villains starring Arrayit Corporation and its president, Mark Schena, whom jurors convicted of swindling investors and running a kickback scheme costing insurers $77 million in false claims for COVID-19 and allergy tests.
The Latest Silicon Valley Lab Testing Scam
Sunnyvale, California-based Arrayit offered diagnostics based on dried finger pricks of blood dropped on a paper card and sent by mail to the company’s lab. Arrayit sought to cash in on the COVID-19 pandemic to sell more of its high-reimbursing allergy tests. In March 2020, as the pandemic was starting, Schena and his colleagues distributed marketing emails claiming that the Sunnyvale, California, company’s unapproved blood test based on finger prick technology was capable of rapid novel coronavirus detection when used in combination with the allergies test kit. According to the criminal complaint filed by the U.S. Department of Justice (DOJ) in June 2020, Arrayit boasted that its tests could work on volumes of blood “250,000 times smaller” than the “nanotainer” infamously employed by Theranos.
Taking a page out of the Elizabeth Holmes playbook, Schena, age 59, also exercised personal charisma to sucker investors, claiming that he was the “father of microarray technology” and on the Nobel Prize shortlist.
Schena also represented the company as having a valuation of $4.5 billion based on annual revenues of $80 million, thanks to the millions of dollars it was generating in billings for its rapid COVID-19 detection test. To further the scheme, Arrayit suppressed publication of its required Securities Exchange Commission (SEC)-required financial disclosure statements and issued press releases and tweets falsely stating that the company had entered into lucrative partnerships with companies, government agencies, and public institutions, including a children’s hospital and a major California healthcare provider, according to the DOJ press release announcing the conviction.
But it was all a pack of lies. Just like the Theranos nanotainer, the Arrayit rapid COVID-19 test didn’t exist. It was only on the day that the emails were sent that Schena actually ordered the antigens. The company later developed and self-validated the test and submitted it to the FDA for Emergency Use Authorization (EUA). But the agency denied EUA clearance after finding the test’s performance wanting.
Arrayit itself was also a sham. The company was broke and on the verge of bankruptcy. But just as with Theranos, the market took the bait. Thinking they had spotted a pearl in the midst of a pandemic, investors caused Arrayit’s stock price to double—albeit, it remained a low-priced penny stock. The SEC suspended trading for the stock.
The DOJ Drops the Hammer
Having sounded the warning on fraudulent coronavirus testing almost from the moment the pandemic started, (See, for example, “OIG Warns of COVID-19 Testing Scams,” LCA, March 30, 2020.), the federal government swooped in on Arrayit. On June 9, 2020, the DOJ charged Schena with criminal securities fraud. And that’s not all.
The government claimed that Schena ran an illegal kickback and healthcare fraud scheme that involved submitting fraudulent claims to Medicare and private insurance for allergy tests that weren’t medically necessary. “Arrayit ran allergy screening tests on every patient for 120 different allergens (ranging from hornet stings to codfish) regardless of medical necessity,” the DOJ stated. It also contended that Arrayit billed more per patient to Medicare for its blood-based allergy tests than any other lab in the US, charging some insurers more than $10,000 per test.
To make matters even worse, Arrayit allegedly paid doctors kickbacks to use their provider numbers to charge insurance companies for patients the doctors had never seen. For example, one doctor told investigators that Arrayit used his provider identifier number to receive insurance reimbursement for allergy tests from patients the doctor had never treated. An Arrayit executive paid the doctor illegal kickbacks from the reimbursement in exchange. Investigators said Medicare paid Arrayit $290,000 and private insurance companies another $2 million for medically unnecessary tests generated as the result of kickbacks.
The Potential Consequences
The jury convicted Schena on 10 counts:
Like Holmes and Balwani, Schena is now awaiting sentencing, which is scheduled to take place on January 30, 2023.
Subscribe to view Essential
Start a Free Trial for immediate access to this article