Beyond the Pap Smear: New Methods to Improve Cytological Screening
According to American Cancer Society (ACS) estimates, roughly 14,140 new cases of invasive cervical cancer will be diagnosed in the US in 2022; and about 4,280 women will die from cervical cancer. These sobering numbers belie the fact that cervical cancer is highly preventable. Conventional cytology screening methods, i.e., Papanicolaou or “Pap” tests, have proven extremely effective in reducing cervical cancer incidence and mortality. But they also require the use of analytical microscopy methods that are prone to yielding inaccurate results. The good news is that researchers and test makers are making significant progress toward developing new and more cost-effective screening methods that can be deployed at the point of care. Pap Testing Pros & Cons The Pap smear is a non-invasive cytologic screening test performed by scraping cells from the cervix, fixing them onto a glass slide, and examining them under a microscope. Because the Pap test identifies precancerous cells or cellular changes in the cervix before they become invasive tumors, it is valuable for screening and early detection, which can improve case outcomes and save lives. Widespread adoption of Pap tests in clinical practice has reduced cervical cancer death rates by nearly 50 percent in American women. However, […]
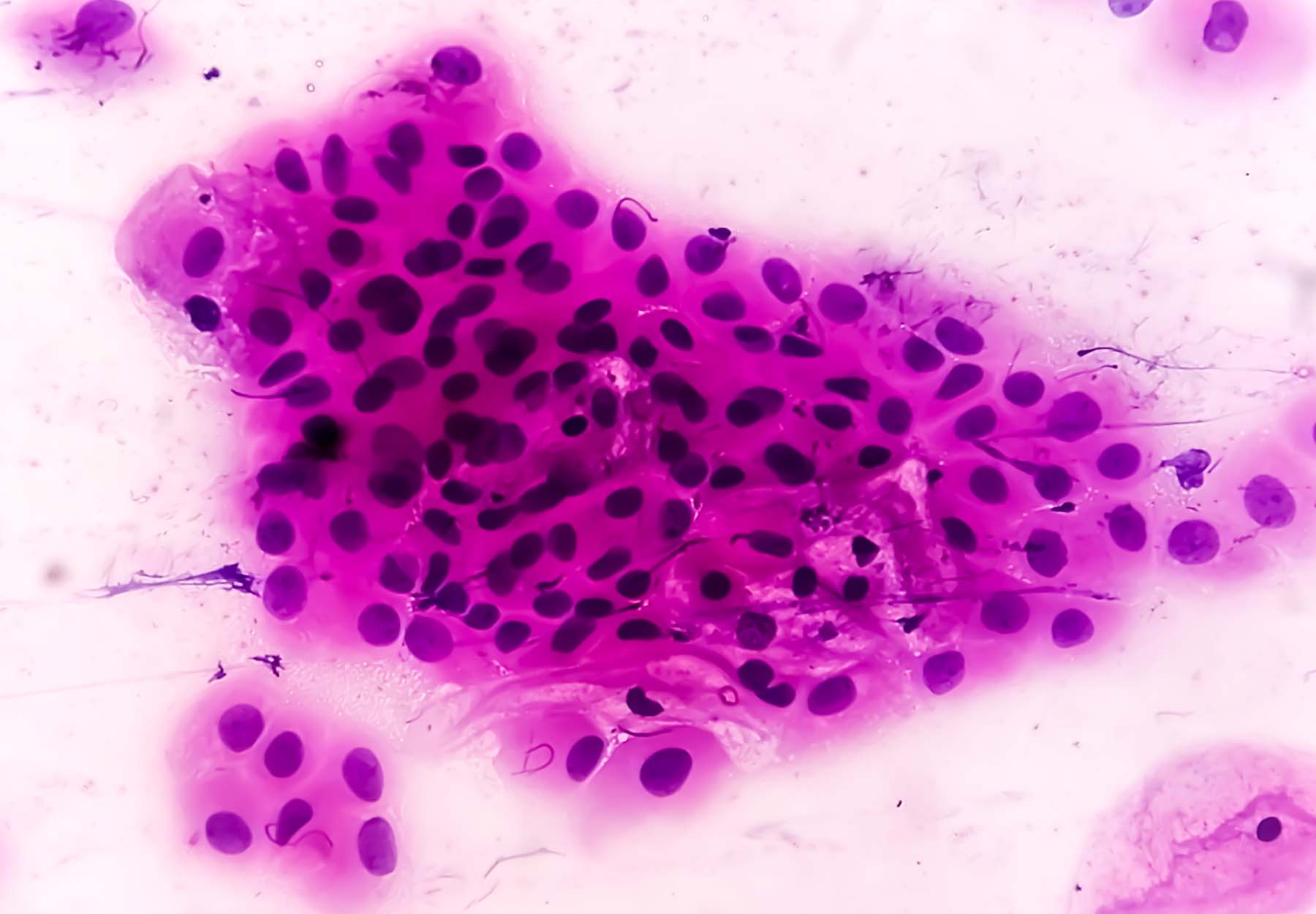
Pap Testing Pros & Cons
The Pap smear is a non-invasive cytologic screening test performed by scraping cells from the cervix, fixing them onto a glass slide, and examining them under a microscope. Because the Pap test identifies precancerous cells or cellular changes in the cervix before they become invasive tumors, it is valuable for screening and early detection, which can improve case outcomes and save lives. Widespread adoption of Pap tests in clinical practice has reduced cervical cancer death rates by nearly 50 percent in American women. However, one drawback to the Pap smear is its low sensitivity, which ranges from 30 to 87 percent. This means that visually examining the cervix using colposcopy or biopsy is necessary to confirm abnormal results. Another problem with Pap testing is its lack of scalability. For many hours each day, skilled professionals must inspect slides under the microscope, sifting through thousands of microscopic objects to find abnormal cells. This need for analysis by medical experts limits the use of Pap testing, especially in rural and resource-limited settings where cytotechnologists and pathologists are not widely available. Relying entirely on human input to interpret Pap smears also makes the process susceptible to errors. Failure to flag abnormal cells introduces false negative errors into the results, which can lead to cervical cancer going undetected in its early non-invasive stage and developing into advanced disease. According to a Clinical Risk research article, even in countries with established cancer screening programs, a variety of factors such as loss of concentration and task fatigue may lead to false-negative rates of five to 10 percent. Conversely, a false positive result may cause women to undergo procedures that are not only medically unnecessary, but also financially and psychologically burdensome. Last but not least, the effectiveness of Pap testing is affected by the frequency of testing, which makes it susceptible to patient non-compliance, such as missed screening appointments. ACS recommends cervical cancer screening with an HPV test alone every five years for everyone with a cervix from age 25 until age 65. If HPV testing alone is not available, people can get screened with an HPV/Pap co-test every five years or a Pap test every three years.Advances in Cytological Screening
The need to develop better and more advanced cervical screening technologies represents a significant opportunity for the diagnostics industry. The money is there. The global market for histology and cytology stood at $12.35 billion in 2020 with expectations of expanding at a compound annual growth rate of 14.74 percent through 2028. Advances in digital cytological screening are proceeding across many different fronts.Digital & AI Cytological Solutions
One approach to improving the accuracy and effectiveness of cytological diagnostics is via automation and integration of digital and artificial intelligence (AI) solutions that enable rapid interpretation of primary diagnostic data. These methods eliminate the need for labor-intensive and subjective interpretation of cellular morphology. Many companies are launching new and advanced digital cytology systems to assist with rapid on-site evaluation of cytology samples. For instance, in February 2021, Hologic teamed up with Google Cloud on a project to use AI and machine learning to improve cervical cancer screening by integrating machine learning technologies into its Genius Digital Diagnostics System.Liquid-Based Cytology
In liquid-based cytology, cellular material collected from the cervix is rinsed and fixed in a vial of preservative fluid before placing it on a slide as a thin layer. This preserves the cellular structure, clears debris, and prevents clumping—addressing issues that preclude the accurate interpretation of morphological features on conventional Pap smears. The technique also allows samples to be transported over long distances for analysis, which is especially beneficial in places without access to trained cytologists (although the relatively high cost of vials, test kits, slides, and other consumables may limit their use in regions with limited resources). There is still debate about whether liquid-based preparations offer significant diagnostic benefits over conventional Pap smears. According to Raeesa Gupta, PhD, in a recent Clinical Lab Manager article, “several studies report that the sensitivity and rate of detection of cellular anomalies is comparable or only slightly higher for liquid-based preparations compared to conventional smears.” However, more recent studies have found that liquid-based preparations yield significant improvements. At the end of the day, most commercial screening systems use liquid-based cytology for sample preparation. The U.S. Food and Drug Administration (FDA) has approved commercial analyzers that use liquid-based cytology for sample preparation and flagged morphological anomalies for human inspection. Many of these analyzers are now commonly used in clinical practice, including Becton Dickinson’s SurePath and FocalPoint GS Imaging systems, and Hologic’s ThinPrep.HPV Testing
The human papillomavirus (HPV) is a group of more than 200 sexually transmitted viruses. HPV testing is performed using PCR amplification to detect DNA or mRNA of high-risk viral genotypes. Currently, there are more than 250 commercial HPV tests available to detect 425 viral variants. HPV co-testing along with a Pap smear has been used to optimize the management of patients with ASC-US, a finding of abnormal cells in the tissue that lines the outer part of the cervix. ASC-US is the most common abnormal finding in a Pap test. Of the patients with ASC-US who undergo colposcopy, only about 20 percent are found to have a high-grade lesion. By identifying patients with a higher risk of developing cervical cancer, HPV co-testing prevents needless testing in low-risk women. The FDA approved the HPV test as a primary screening test for cervical cancer in 2003, and it has been shown to have a higher sensitivity of 95 percent for detecting high-grade cervical intraepithelial neoplasia (CIN), versus 55 percent with the Pap test. Other benefits of HPV testing include high throughput, high reproducibility, low training requirements, and high negative predictive value. DNA Methylation Testing DNA methylation tests offer another potential method for improving cervical cancer screening and risk stratification to the extent that epigenetic silencing of tumor suppressor genes via DNA methylation is implicated in the progression of cervical cancer. In other words, high-grade lesions have significantly higher rates of DNA methylation versus low-grade lesions. Methylation assays are relatively easy to set up, perform, and automate. Testing can even be performed directly from self-collected specimens. A recent study from China published in the June 2020 volume of Clinical Epigenetics found that methylation testing achieved a success rate of 98.4 percent (306/311) when using residual cytology samples stored during the previous year. What’s more, a recent meta-analysis that included 16,336 women from 43 studies found that, as a screening test, DNA methylation had a higher sensitivity than some HPV DNA tests and a higher specificity than cytology testing. It’s clear this technology, though new, has the potential to improve cervical cancer screening.Takeaway
While the Pap smear still represents the gold standard in cervical cancer screening, its dependence on intensive microscopy analysis by medical experts and susceptibility to error pose major obstacles to its wider use, particularly in rural and low-income locations. But promising new advances offer the possibility of overcoming the limitations of Pap tests. These potential solutions encompass everything from AI and machine learning to the deployment of HPV and other forms of non-cytological testing. Editor's Note: This story is based on the article “Advances in Cervical Cancer Screening: What Lies Beyond the Pap Smear?” by Raeesa Gupta, PhD, that was published in the November 9, 2021 issue of our sister publication, Clinical Lab Manager.Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article