December 2022 FDA Watch: More Approvals in US than EU
A high volume of FDA approvals for new tests and diagnostic products made up for the relatively low number of approvals in the EU.
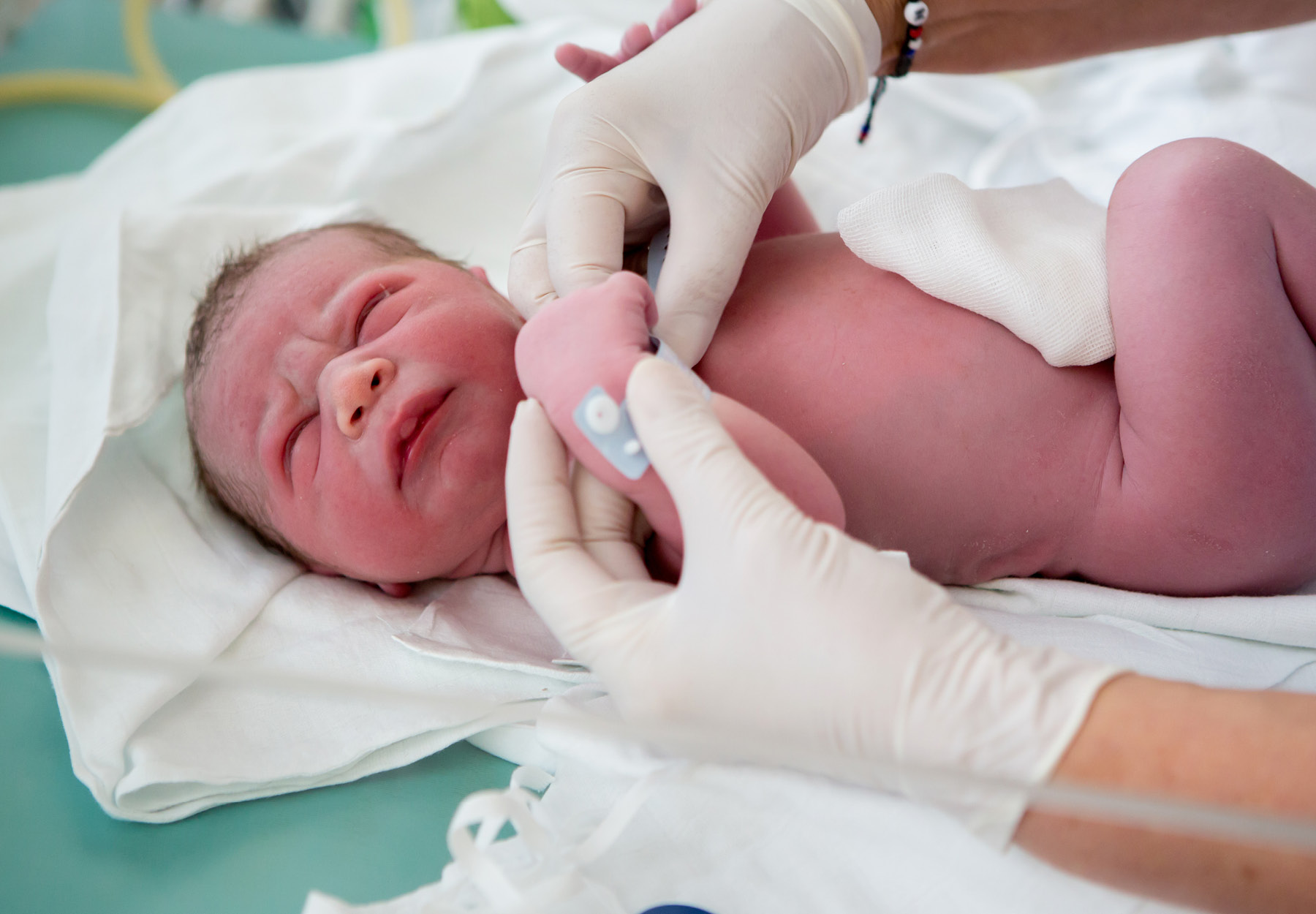
Regulators Approve 3 Breakthrough Newborn Screening Products in US and Europe
Higher volume of FDA clearances of new laboratory test and diagnostics products in the US made up for the relatively low number of approvals in the EU, which has now transitioned to its new and somewhat controversial In Vitro Diagnostic Regulation (IVDR) regime. One of the biggest stories of November was the three novel newborn screening products to secure first of their kind regulatory approval in Europe or the US. Here’s a quick briefing on each newborn testing product.
AI Severe Respiratory Disease Platform (SIME Diagnostics)
On Nov. 14, SIME Diagnostics announced it received CE-IVD accreditation in Europe for what it claims is the world’s first clinical AI platform for rapid prediction of severe respiratory disease. The device uses proprietary algorithms to analyze thousands of unique datapoints associated with severe respiratory disease in less than 15 minutes. The UK firm, which produces point-of-care respiratory diagnostics for the ICU, has launched a pilot called RDS Predict to rapidly identify which newborns are likely to develop respiratory distress syndrome (RDS), the leading cause of morbidity and mortality in babies born prematurely.1
Eonis SCID-SMA Simultaneous Detection Kit for Newborn Screening (PerkinElmer)
PerkinElmer secured FDA clearance to market an assay that simultaneously detects spinal muscular atrophy (SMA) and severe combined immunodeficiency (SCID). The Eonis SCID-SMA assay kit is the first SMA screening assay for newborns to receive FDA marketing authorization, according to the company. The kit had to travel two pathways to reach the US market, with the SMA portion of the test receiving De Novo and the SCID portion getting 510(k) clearance from the agency. The kit is designed for use with dried blood spot samples on PerkinElmer’s Eonis real-time PCR instrument.2
Congenital CMV Kit for Saliva and Urine Samples (DiaSorin)
On Nov. 9, DiaSorin announced that its Simplexa Congenital CMV Direct kit for detection of cytomegalovirus (CMV) had received 510(k) clearance from the FDA. Congenital CMV is a potentially fatal condition that can occur when a mother passes the virus to her child. Run on DiaSorin’s Liaison MDx platform, the Simplexa assay detects DNA from the virus in newborns up to 21 days old. It’s the first product cleared by the FDA for diagnosing congenital CMV from both saliva swab and urine specimens, according to the Italian testing giant. The kit received CE clearance for marketing in Europe in 2020.3
References:
- https://www.prnewswire.co.uk/news-releases/sime-ai-device-that-predicts-acute-respiratory-disease-receives-ce-mark---changing-the-standard-of-care-for-millions-of-babies-worldwide-301675729.html
- https://www.businesswire.com/news/home/20221114005255/en/PerkinElmer-Announces-its-EONIS-SCID-SMA-Kit-is-First-to-Receive-Marketing-Authorization-by-U.S.-FDA-for-SMA-Screening-in-Newborns
- https://diasoringroup.com/sites/diasorincorp/files/allegati_pressrel/diasorin_simplexar_ccmv_direct_assay_received_u.s._fda_510k_clearance.pdf
****
Here are some of the key new FDA EUAs and clearances that were announced in November 2022:
New FDA Approvals & Emergency Use Authorizations (EUAs)
New CE Marks & Global Certifications
Notable European CE certifications announced during the period:
New CE Markings in Europe
Other international clearances announced during the period:
Subscribe to view Essential
Start a Free Trial for immediate access to this article