FDA Sounds the Warning on Use of Unauthorized SARS-CoV-2 Rapid Tests
COVID-19 tests reaching the US market without proper Food and Drug Administration (FDA) authorization has been a concern almost from the moment the public health emergency began. After an initial crackdown, the agency seemed to have gotten the problem under control. But with rising demand for rapid, point of care tests spurred by the Omicron outbreak, the FDA gateway seems to have sprung another leak, producing a new stream of warning letters not seen since the early days of the pandemic. The Latest Round of FDA Warnings On March 1, the agency issued Safety Communications warning providers and consumers not to use unauthorized tests. The good news is that the agency indicated that it hasn’t received reports of any actual deaths, injuries, or other adverse health consequences associated with use of the products. However, because they’re unauthorized and haven’t gone through FDA vetting, they pose relatively high risk of producing false test results. The agency issued separate warnings covering tests from three different companies: The Celltrion DiaTrust COVID-19 Ag Rapid Test, which uses a mid-turbinate nasal swab sample to detect SARS-CoV-2. What makes this situation particularly tricky is that the company that produces the test, Celltrion HealthCare, also sells two […]
The Latest Round of FDA Warnings
On March 1, the agency issued Safety Communications warning providers and consumers not to use unauthorized tests. The good news is that the agency indicated that it hasn’t received reports of any actual deaths, injuries, or other adverse health consequences associated with use of the products. However, because they’re unauthorized and haven’t gone through FDA vetting, they pose relatively high risk of producing false test results. The agency issued separate warnings covering tests from three different companies: The Celltrion DiaTrust COVID-19 Ag Rapid Test, which uses a mid-turbinate nasal swab sample to detect SARS-CoV-2. What makes this situation particularly tricky is that the company that produces the test, Celltrion HealthCare, also sells two tests that have received Emergency Use Authorization (EUA) and which the warning doesn’t cover. One of those products actually has the same name as the unauthorized test. The difference is in the packaging. The test that the agency says should not be used is the one that comes in a green and white box.
The Celltrion DiaTrust COVID-19 Ag Rapid Test. Image provided by the FDA.
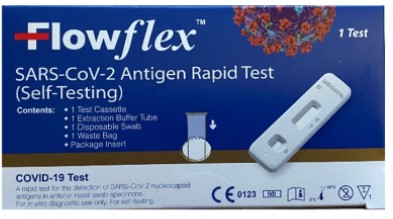
The ACON Laboratories Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing), which comes in a dark blue box. Image provided by the FDA.
The Previous FDA Warnings
Not even three months into the year, the FDA has issued no fewer than six separate warnings covering eight different unauthorized SARS-CoV-2 rapid tests (seven antigen and one antibody), more than quadrupling the total warnings issued for all types of unauthorized COVID-19 tests in 2021. On Jan. 11, the agency posted a warning for two unauthorized tests from LuSys Laboratories: the LuSys Laboratories COVID-19 Antigen Test (Nasal/Saliva) and LuSys Laboratories COVID-19 IgG/IgM Antibody Tests. The tests have also been sold under the names Luscient Diagnostics or Vivera Pharmaceuticals, or with the trade name EagleDx, for use in laboratories or at-home testing, according to the agency. On Jan. 28, the FDA issued a warning for a pair of rapid COVID-19 tests from Empowered Diagnostics: the CovClear COVID-19 Rapid Antigen Test, and the ImmunoPass COVID-19 Neutralizing Antibody Rapid Test. Florida-based Empowered is recalling both tests, in what the FDA has classified as a Class 1 recall, the most serious type. The third Safety Communication of the year came out on Feb. 4 warning against the use of an unapproved test from Cambridge, Massachusetts laboratory E25Bio called the E25Bio COVID-19 Direct Antigen Rapid Test (DART test). The test may also be sold under the trade name E25Bio SARS-CoV-2 Antigen Test Kit. In addition to risks of false results, the agency expressed concern that the DART test was sold directly to consumers with labeling instructing users to collect a sample from deep inside the nose, reaching the back of the throat (nasopharyngeal) or from the middle part of the throat (pharynx) just beyond the mouth (oropharyngeal). Self-collecting nasopharyngeal or oropharyngeal samples for SARS-CoV-2 testing could result in serious injury when not done by trained professionals, the agency cautions. The FDA says that users who’ve been tested with any of the warned-of assays should talk to their health care providers and that providers that offered the antigen test less than two weeks ago (or the antibody test at any time) should consider retesting their patients.Subscribe to view Essential
Start a Free Trial for immediate access to this article