Feds Lower the Boom on Home Sleep Test False Billing and Kickbacks
A recent $3.5 million settlement illustrates the kinds of pitfalls that can result in liability for hospital and freestanding sleep labs.
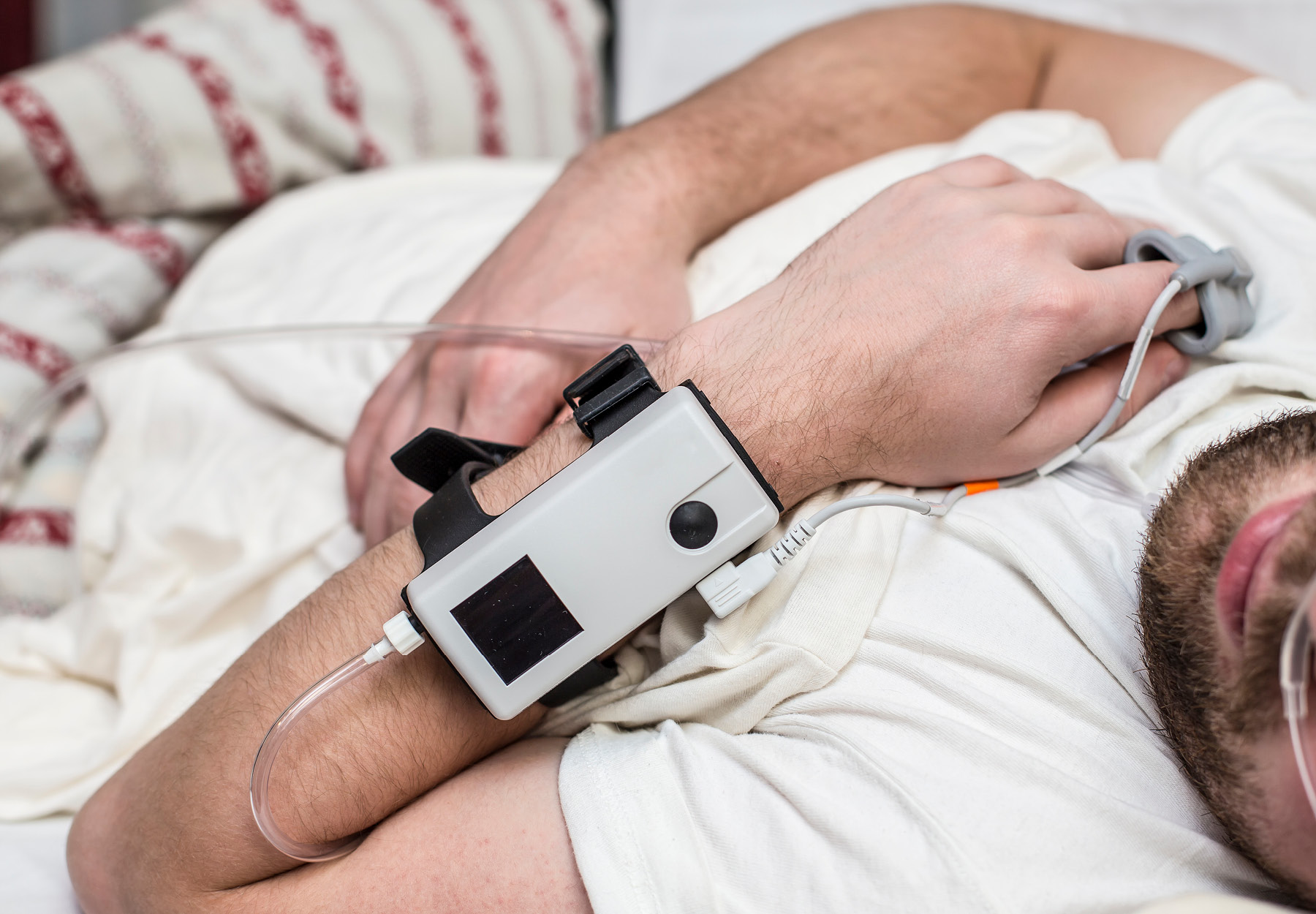
While documenting medical necessity of lab tests is a crucial part of compliance, it can be tricky for labs that perform home sleep studies to diagnose patients for apnea. A recent $3.5 million settlement illustrates the kinds of pitfalls that can result in liability and that compliance officers of hospital and freestanding sleep labs must be on guard for.
The Context: What Sleep Testing Is All About
An estimated 10 percent to 30 percent of Americans suffer obstructive sleep apnea (OSA), a disorder that causes breathing to stop and start during sleep. The principle means of diagnosing OSA and evaluating the effectiveness of using a positive airway pressure (PAP) device to manage it is polysomnography, a sleep study in which patients sleep overnight while connected to sensors measuring brain waves, blood oxygen flow, and other parameters.
When the polysomnography indicates that a patient has a sleep disorder, the lab will often conduct a PAP titration study as well. Patients may also receive a PAP device for home use after the titration study. In so-called split-night cases, both studies are performed on the same patient on the same night—the polysomnography followed a few hours later by the PAP titration.
As awareness of OSA increases, home sleep testing has grown in popularity. Not surprisingly, this increase in utilization has been accompanied by more intense regulatory scrutiny.
Medicare Billing of Sleep Tests
The first risk area associated with polysomnography is Medicare billing and coding. The most common code for billing sleep order tests is HCPCS 95810. As with most diagnostic services, polysomnography consists of two components:
- The technical component covering administration of the test, signified by HCPCS modifier code -TC; and
- The professional component covering the provider’s interpretation of the test results, signified by code -26.
Failure to list either modifier is an indication that the lab is billing globally for both the technical and professional components. HCPCS 95811 is the code for both PAP titration and split-night services.
The $3.9 Million SNAP Diagnostics Settlement
False billing of home sleep tests has been on the radar of federal fraud enforcers and whistleblowers for nearly a decade. The most recent case began when whistleblowers filed a qui tam lawsuit against Chicago area-based national sleep lab SNAP Diagnostics and two of its officers. The whistleblowers claimed that the officers directed SNAP to submit claims for Medicare patients’ second and third nights of home sleep testing knowing that it needed only one night of testing to effectively diagnose OSA and that it routinely tested and claimed only one night for privately insured patients. In addition to defrauding five federal agencies, they accused SNAP of illegally multiplying the copays it received from Medicare beneficiaries.
Other Enforcement Actions Against Sleep Labs
The next big case came down in 2016, targeting Florida clinic Sleep Health. The OIG audited 100 random Medicare patients who had received polysomnography services over two years. The findings, listed in the OIG’s September 2016 report: The Fort Myers-based clinic received $48,934 in overpayments—$141,339 in total when extrapolated over the three-year recovery period. In addition to paying back that money, the OIG called on Sleep Health to work with the Medicare Administrative Contractor (MAC) to return the estimated $345,593 in overpayments it allegedly received outside the three-year recovery period.
SNAP vigorously denied the allegations. But when the federal government decided to intervene, the pressure to settle intensified. Under the settlement, SNAP will pay $3.925 million, with $300,000 coming from the company’s founder and $125,000 from its vice president. SNAP also has to enter into a corporate integrity agreement requiring the firm to hire an independent organization to perform an annual review of claims and submit reports to the OIG.
Ensure Proper Billing and Documentation of Sleep Tests
According to a Medicare local coverage determination, before sleep testing is performed, two sets of documentation are required:
The treating physician must perform a face-to-face clinical evaluation documenting the need for testing that includes a minimum of three things:
- The patient’s symptoms and sleep history;
- An Epworth sleepiness scale; and
- A focused cardiopulmonary and upper airway evaluation.
The treating physician must then write an order for the study.
The lab that performs the study must keep a record of the attending physician’s face-to-face evaluation and written order.
Beware of Sleep Testing Kickbacks
Sleep test arrangements may also raise red flags under kickback laws. Thus, the whistleblowers claimed that SNAP paid physicians kickbacks for home sleep testing referrals. The three kinds of kickback arrangements the complaint cites involve common kinds of illegal remuneration that get labs into hot water, including allegedly:
- Offering to interpret certain sleep tests of commercially insured patients and then providing the referring physicians an unsigned report—essentially a blank check that physicians could sign and use to seek reimbursement for the professional component of the test;
- Using an independent contractor sales force but paying it volume-based commissions; and
- Offering to waive the copays on sleep tests for providers and members of their family or staff.
Subscribe to view Essential
Start a Free Trial for immediate access to this article