New and more generous Medicare Part B lab specimen collection fees take effect on January 1, 2023. Here’s a quick briefing on the Centers for Medicare & Medicaid Services’ (CMS’s) final rule and what labs will need to know to properly bill specimen collection fees.
The New Laboratory Specimen Collection Fee Regulation
Previously, CMS requirements for billing and payment of specimen collection fees and travel allowances were contained not in the regulations but the Medicare Claims Processing Manual Pub. 100-04, chapter 16 §§ 60.1-.2. CMS has also issued Medicare transmittals explaining the rules. However, there’s been some inconsistency between the manual and the transmittal guidance. The travel allowance rules were also confusing and out of touch with actual healthcare practices. To address these issues, the final rule includes a new regulation, 42 C.F.R. § 414.523, aka, “Payment for laboratory specimen collection fee and travel allowance.”
Eligibility: The new regulation doesn’t change the eligibility criteria governing which specimens for clinical lab testing are billable. As before, labs may only bill Medicare for lab test specimens that are:
- Used to perform a Medicare-covered lab test that’s paid under the Part B Clinical Laboratory Fee Schedule (CLFS);
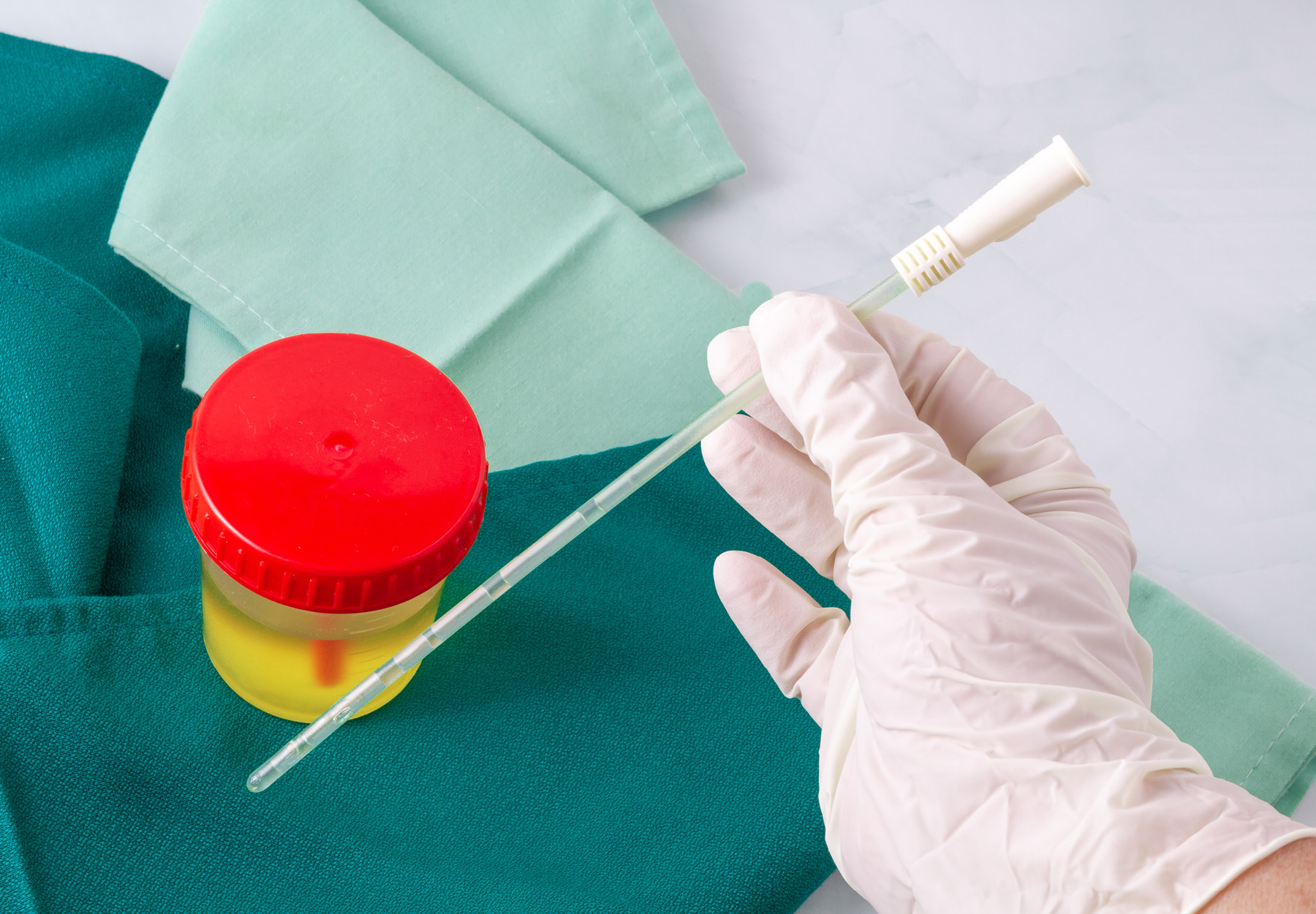
- Collected by a trained technician from a Medicare beneficiary who’s either: i.) homebound; or ii.) a non-hospital inpatient at a facility where no qualified personnel are available to collect the specimen; and
- A blood specimen collected via venipuncture or a urine sample collected by catheterization.
Billing Restrictions: Making clear what was previously only implied, the final rule specifically states that the above are the only circumstances in which Medicare will pay a specimen collection fee. Clearing up confusion caused by inconsistency between Medicare requirements and U.S. Bureau of Labor Statistics definitions, the final rule also explains that “trained technician” for purposes of the second criterion means “staff providing specimen collection services,” including phlebotomists. CMS also says it will stick to its previous policy of paying only one specimen fee per single patient encounter, even if different types or multiple specimens are drawn from the patient.
Rates: CMS initially proposed to keep specimen collection fees at the current rates of $3 per patient or $5 per patient in a skilled nursing facility (SNF) or being serviced by a home health agency (HHA). However, in response to public comments, the agency agreed to revise the rates for inflation. The new rates:
- $8.57 for all specimens collected in a single qualifying patient encounter; and
- $10.57 per beneficiary in an SNF or being serviced by an HHA.
More good news: Starting January 1, 2024, CMS will update the specimen collection fee rate every calendar year by the percentage change in the Consumer Price Index for All Urban Consumers (CPI-U) for the 12 months ending June 30 for the preceding year. Learn more in an upcoming article in the January 2023 National Lab Reporter.