Keep Informed on Legal and Compliance Developments Affecting Diagnostic Labs
Best practices and expert insight to help lab professionals comply with regulations and identify strategic trends in business and technology
Already a Subscriber? Log in Here
Lab Industry Advisor Subscription
Struggling with Staying Informed? In the fast-paced world of diagnostic medicine, staying updated with the latest legal, compliance, regulatory, and industry insights is crucial. Yet, finding reliable and comprehensive information can be overwhelming and time-consuming.
Lab Industry Advisor to the Rescue! Lab Industry Advisor provides comprehensive, up-to-date insights and analyses tailored to your needs. With expert commentary and actionable advice, you’ll stay ahead of the curve. No more sifting through endless sources—everything you need is right here!
Start your free trial today and see how Lab Industry Advisor can transform your approach. Get instant access to invaluable resources designed to keep you informed and competitive. Explore detailed reports, in-depth articles, and exclusive industry insights that help you make better decisions faster. Choose from three subscription levels tailored to fit your needs: Essential, Premium, and Elite.






Additional Business Intelligence Offerings
Lab Industry Advisor Subscriptions Include

Analysis and insight on key compliance, legal, and business developments affecting laboratories
- Updates on changes to regulations
- Healthcare-related laws
- FDA approvals
- Business deals in the diagnostics space, and more
Latest Articles
Routine Mass Spectrometry in Clinical Labs: A Competitive Advantage?
How a new automated system bringing MS to routine lab testing could help set laboratories apart from their competitors
Dx Deals: BD Laboratory Business Is Up for Grabs in Bold Move
Becton Dickinson divisions responsible for $3.4 billion in annual revenue are on the block
Updates to CLIA Personnel Requirements May Lengthen Hiring Times
Changes ‘have had a significant impact on the recruitment process for laboratory directors and technical supervisors,’ recruiter says
Clinical Lab Efficiency Gains May Be Best Achieved Through a Tailored Approach
Optimizing efficiency unfortunately is not straightforward in medical laboratories
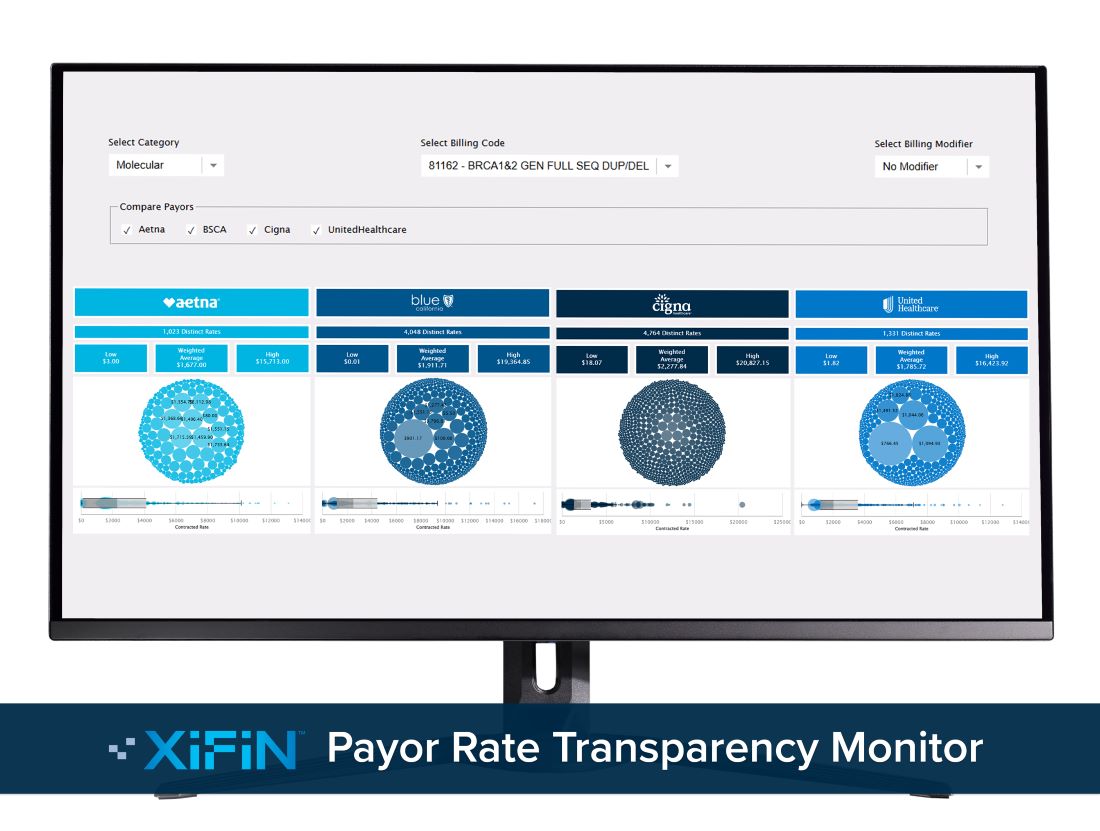
XiFin’s Payor Rate Transparency Monitor Helps Clinical Labs Compare In-Network Rates
The free online tool can set a foundation for renewed reimbursement strategies and contract negotiations with insurance carriers
Recent Decision in FCA Case Has Key Implications for Labs
The decision deepens a split among circuit courts regarding how to determine liability for false claims
MeMed Sepsis Test Gains Breakthrough Device Designation
The 15-minute assay to detect sepsis and assess its severity and risk is expected to get full FDA approval by 2026
Labcorp, Quest, and ARUP Develop Bird Flu Tests with CDC Help
The assays were developed with the CDC as part of recent trend of public-private partnerships to improve pandemic preparedness
Implementing Accessible On-Demand Testing: A Lab Director’s Experience
On-demand testing doesn’t require registration, billing, or scheduling, keeping costs low while collecting “real” dollars upfront
Dx Deals: Could Health Coaching Be a New Opportunity for Labs?
Quest Diagnostics will find out as it launches a new health coaching business that relies on lab testing, via its Pack Health subsidiary
Reimbursement Top Challenge for Labs in 2025
ACLA president Susan Van Meter discusses some of the key issues labs faced in 2024, along with work to address these developments through 2025