Screening Shortfalls and Self-Collected Samples
Is at-home sample collection the solution to lagging cancer screening uptake—or does this approach introduce a new set of challenges?
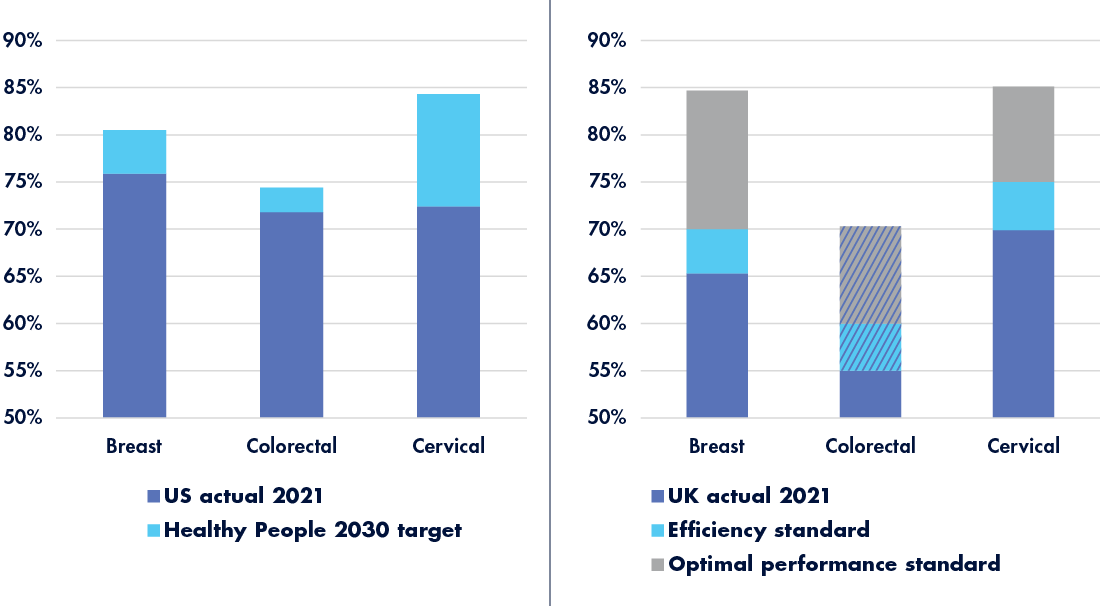
It’s no secret that uptake is a challenge for cancer screening programs. Although it’s estimated that perfect adherence to screening guidelines in the United States could save as many as 21 million lives1—not to mention up to $11.3 trillion in healthcare costs—screening numbers remain consistently below targets (see Figure 1).
Patient factors affecting screening uptake vary widely,4 but consistent influences include:
- demographic factors such as age, gender, or ethnicity
- socioeconomic factors including time, resources, and access
- cultural factors such as social norms and stigmas
- health literacy, especially perceived risk and efficacy of screening
- fear of the screening procedure or its results
- fear of the potential treatment needed if a cancer diagnosis is made
- embarrassment or disgust surrounding the screening procedure
Removing barriers to colorectal cancer screening
A number of these factors can be mitigated by at-home screening. People who struggle with time, transportation, or access to healthcare5 can receive test kits by mail rather than attend an in-person appointment. Those who experience fear or judgment in healthcare settings6 can collect their samples in a more comfortable environment. Noninvasive testing is less likely to induce anxiety7 and patients often feel less fear when in control of their own sample collection. And although it may not be possible to remove all feelings of embarrassment or disgust,8,9 these may be milder when patients are able to complete their sample collection privately, rather than undergo a procedure in the presence of healthcare providers.
But do at-home testing options increase screening rates? It seems so; Norwegian researchers who compared on-site sigmoidoscopy and home fecal immunochemical testing (FIT) uptake over 10 years found that the at-home option achieved approximately 60 percent participation, whereas the on-site test achieved only 52 percent—despite the fact that FIT testing was required every two years and sigmoidoscopy took place only once.10 The study also found that overall participation was lower in more deprived populations, but that members of these populations were more likely to accept FIT testing than sigmoidoscopy—a result borne out by similar studies in the UK.11, 12 At-home testing alone may not be sufficient to achieve screening targets, but combining it with mass media campaigns,13 targeted health education,14 and other approaches such as providing screening kits alongside annual vaccines and other regular appointments15 has the potential to significantly increase uptake.
Removing barriers to cervical cancer screening
Although there’s no at-home Pap testing, many health systems are now implementing human papillomavirus (HPV) testing as an alternative cervical cancer screening option. Research suggests that self-collected samples may yield lower sensitivity and specificity than those taken by clinicians,16 but remain a viable testing strategy, especially for people who might not otherwise participate in screening. A recent study in Italy telephoned people who were overdue for Pap screening to offer them an alternative HPV test with at-home sample collection. Of the 500 individuals who opted into the study, 400 (80 percent) returned a sample—far exceeding rates from prior studies that used an opt-out format—and 319 (64 percent) did so without requiring a reminder.17 A US-based clinical trial involving 31,355 people further supported the ability of self-collection to increase screening uptake and found direct outreach—in this case via mail—significantly more effective than education alone, increasing participation by more than 14 percent over routine care.18 Research indicates that, in general, at-home testing achieves higher participation rates when supported by intensive outreach efforts.19
A truly effective intervention?
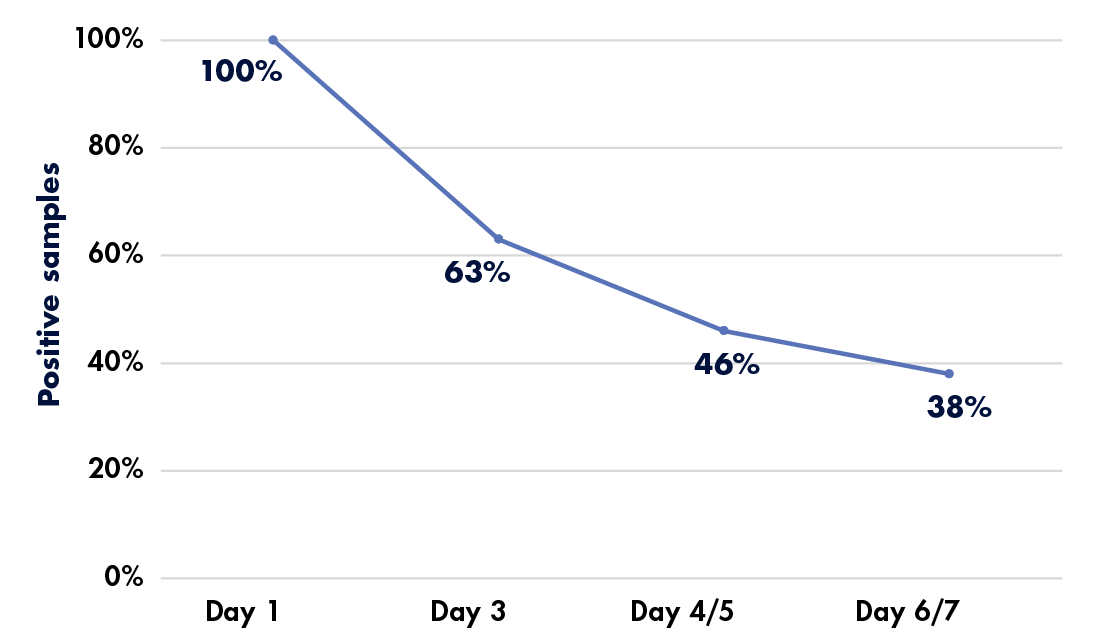
Although at-home sample collection may increase rates of participation in cancer screening programs, it also introduces challenges that may not be present in the clinic. Sample quality and handling may not be satisfactory, as demonstrated by a recent study’s findings that over 10 percent of self-collected fecal immunochemical test (FIT) samples could not be processed for reasons ranging from an inadequate specimen to an incomplete label.20 Shipping presented another potential difficulty, with 8 percent of samples arriving in broken or leaking containers. “The fact that, in most instances, unsatisfactory FIT was not followed by a timely subsequent test highlights the need for systems to have a better, more comprehensive approach to tagging and following up unsatisfactory FIT,” said study co-first author Po-Hong Liu, MD, MPH, in a press release.21 FIT specimens also lack stability; research indicates that hemoglobin concentration in the samples decreases over time, even when refrigerated, potentially affecting results (see Figure 2) and follow-up care if sample delivery is delayed.22
To address incorrect sample handling by non-experts, researchers in the UK trialed a fecal collection device to simplify at-home testing.23 Unfortunately, of the 429 participants who used the device at least once, only 38.4 percent found it helpful and 31.5 percent reported that it made the task more pleasant. A surprising 44.6 percent found that it made collection more difficult and 25 percent considered sample collection less pleasant using the device—making widespread adoption unlikely.
HPV testing faces similar challenges; not all assays that offer at-home sample collection control for specimen adequacy,24 which can lead to inconclusive or false-negative results. Even when successful, however, increasing screening rates may not equal a victory in the war against cancer. Less than half of patients who receive atypical results—whether via Pap smear25 or HPV testing26—seek out follow-up care, especially those who are members of minority or low socioeconomic status groups. It’s not enough to overcome screening barriers; at-risk populations need adequate samples, accurate results, and increased access to every step of the diagnostic process to enable early cancer detection and improve outcomes.
References:
- Philipson TJ et al. The aggregate value of cancer screenings in the United States: full potential value and value considering adherence. BMC Health Serv Res. 2023;23(1):829. doi:10.1186/s12913-023-09738-4.
- Online Summary of Trends in US Cancer Control Measures: Early Detection. National Cancer Institute. August 2023. https://progressreport.cancer.gov/detection.
- Cancer screening. Nuffield Trust. June 27, 2023. https://www.nuffieldtrust.org.uk/resource/breast-and-cervical-cancer-screening.
- Young B, Robb KA. Understanding patient factors to increase uptake of cancer screening: a review. Future Oncol. 2021;17(28):3757–3775. doi:10.2217/fon-2020-1078.
- Lee KMN et al. Distance and Transportation Barriers to Colorectal Cancer Screening in a Rural Community. J Prim Care Community Health. 2023;14:21501319221147126. doi:10.1177/21501319221147126.
- Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012;23(4 Suppl):67–76. doi:10.1353/hpu.2012.0148.
- Austin KL et al. Perceived barriers to flexible sigmoidoscopy screening for colorectal cancer among UK ethnic minority groups: a qualitative study. J Med Screen. 2009;16(4):174–179. doi:10.1258/jms.2009.009080.
- Bynum SA et al. Unwillingness to participate in colorectal cancer screening: examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. Am J Health Promot. 2012;26(5):295–300. doi:10.4278/ajhp.110113-QUAN-20.
- Davis M et al. An investigation of the emotion of disgust as an affective barrier to intention to screen for colorectal cancer. Eur J Cancer Care (Engl). 2017;26(4). doi:10.1111/ecc.12582.
- Berstad P et al. Inequalities in colorectal cancer screening uptake. Tidsskr Nor Laegeforen. 2023;143(5). doi:10.4045/tidsskr.22.0760.
- von Wagner C et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011;40(3):712–718. doi:10.1093/ije/dyr008.
- Moss S et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut. 2017;66(9):1631–1644. doi:10.1136/gutjnl-2015-310691.
- Lofti-Jam KL et al. Increasing bowel cancer screening participation: integrating population-wide, primary care and more targeted approaches. Public Health Res Pract. 2019;29(2):2921916. doi:10.17061/phrp2921916.
- Weller DP, Campbell C. Uptake in cancer screening programmes: a priority in cancer control. Br J Cancer. 2009;101 Suppl 2:S55–S59. doi:10.1038/sj.bjc.6605391.
- Potter MB et al. The effectiveness of the FLU-FOBT program in primary care a randomized trial. Am J Prev Med. 2011;41(1):9–16. doi:10.1016/j.amepre.2011.03.011.
- Arbyn M et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi:10.1016/S1470-2045(13)70570-9.
- Feltri G et al. Evaluation of self-sampling-based cervical cancer screening strategy using HPV Selfy CE-IVD test coupled with home-collection kit: a clinical study in Italy. Eur J Med Res. 2023;28(1):582. doi:10.1186/s40001-023-01263-8.
- Winer RL et al. Strategies to increase cervical cancer screening with mailed human papillomavirus self-sampling kits. JAMA. 2023;330(20):1971–1981. doi:10.1001/jama.2023.21471.
- Pretsch PK et al. Effect of HPV self-collection kits on cervical cancer screening uptake among under-screened women from low-income US backgrounds (MBMT-3): a phase 3, open-label, randomised controlled trial. Lancet Public Health. 2023;8(6):e411–e421. doi:10.1016/S2468-2667(23)00076-2.
- Liu P-H et al. Unsatisfactory fecal immunochemical tests for colorectal cancer screening: prevalence, reasons, and subsequent testing. Cancer Epidemiol Biomarkers Prev. 2023; online ahead of print. doi:10.1158/1055-9965.EPI-23-0507.
- More Than 10% of Samples From a Stool-based Colorectal Cancer Test May Be Unsatisfactory. American Association for Cancer Research. November 15, 2023. https://www.aacr.org/about-the-aacr/newsroom/news-releases/more-than-10-of-samples-from-a-stool-based-colorectal-cancer-test-may-be-unsatisfactory.
- Reid MS et al. Evaluation of the stability of fecal immunochemical test specimens. Clin Biochem. 2023;115:92–96. doi:10.1016/j.clinbiochem.2022.11.015.
- Morling JR et al. Could stool collection devices help increase uptake in bowel cancer screening programmes? J Med Screen. 2018;25(4):174–177. doi:10.1177/0969141317753463.
- Aptima HPV Assay. Hologic. April 2017. https://www.hologic.com/sites/default/files/package-insert/AW-14517-001_003_01.pdf.
- Benard VB et al. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol. 2005;105(6):1323–1328. doi: 10.1097/01.AOG.0000159549.56601.75.
- Wang R, Coleman JS. The HPV self-collection paradox: boosting cervical cancer screening, struggling with follow-up care. Lancet Public Health. 2023;8(6):e394–e395. doi:10.1016/S2468-2667(23)00094-4.
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article