Special Report: The 2024 Lab Enforcement Landscape
Lab leaders should be wary of telemedicine arrangements, certain marketing firms, and unnecessary PGx and CGx testing

Top lab compliance enforcement trends in the first half of 2024, as identified by two major healthcare law firms, should interest clinical laboratory leaders because they are not headline-grabbing offenses.
Instead, these trends might be better characterized as stemming from mundane occurrences in labs that, left unchecked, can raise the hackles of the U.S. Department of Justice (DOJ) and the U.S. Department of Health and Human Services’ Office of Inspector General (OIG). As such, compliance risks that labs should be carefully monitoring include the following:
-
- Billing the Medicare program for medically unnecessary diagnostic tests.
-
- Making commission-based payments for lab test business.
- Establishing improper relationships between a lab and telehealth provider.
Meanwhile, some labs that found themselves in the crosshairs of prosecutors were able to lighten their compliance burden by being more cooperative with authorities and self-disclosing problems ahead of time.
This special report will look into more detail on each of these matters.
DOJ crackdown on medically unnecessary PGx and CGx tests
In terms of medical necessity, labs should be wary when seeking reimbursement for two types of tests, says attorney Danielle Tangorre, a partner with the firm Robinson+Cole:
-
- Pharmacogenomic (PGx) tests, which help physicians avoid prescribing medication that may cause adverse reactions in certain patients.
- Cancer genetic (CGx) testing, which detects genetic variants that could indicate a higher chance of cancer in certain patients.
“In prior years, OIG reports raised concerns about the increased expenditure of PGx and CGx testing,” Tangorre notes. “The DOJ has now adopted those concerns as enforcement priorities, which resulted in several criminal indictments this year.”
Many of these cases include some level of kickback allegations, too, says attorney Samantha Kingsbury, of counsel at law firm Mintz.
“This year we have seen numerous criminal and civil actions involving individuals and entities associated with labs accused of performing and billing for genetic testing that was medically unnecessary and/or ordered by a provider who never treated or met the patient in whose name the testing was ordered,” Kingsbury observes. “These cases often include kickback allegations as well.”
As Lab Industry Advisor has noted in past reporting, labs that seek reimbursement for PGx tests should verify that the physician has considered medications that are medically necessary for a patient and have a known gene-drug interaction.1
Compliance tip: Clinical laboratories should document this important verification, such as through internal audits. Lab managers should “ensure that the lab is doing its part in promoting ordering of medically necessary testing and auditing test requisitions for medical necessity,” advises Seth Orkand, who is also a partner with Robinson+Cole.
Commission-based payments prove problematic
The DOJ and OIG have long railed against any kind of inducement that might steer lab test business into murky areas.
Arrangements that potentially violate the Anti-Kickback Statute—such as those involving some third-party marketing and sales contractors who receive commissions for their services—have received attention over the past year, says Karen Lovitch, chair of Mintz’s Health Law Practice and co-chair of the firm’s Health Care Enforcement Defense Practice.
The Anti-Kickback Statute broadly prohibits payments made to induce referrals of business that can be billed to Medicare or other federal healthcare programs. The inducements can include sales and marketing services in which the volume of tests plays into compensation for those services, as well as labs providing services below fair market value in exchange for something.
“With respect to potential arrangements with third parties, a lab should research those third parties to the greatest extent possible to try and confirm that the company is reputable,” Lovitch says. “With respect to arrangements with referral sources, in particular, a lab should conduct its own compliance review to ensure that the arrangement can be executed in a way that does not create legal or compliance risk.”
Lab leaders should note the large number of settlements and enforcement actions in 2023 and 2024 related to commissions paid by laboratories to contracted marketers, the Robinson+Cole team explains. “Labs should avoid paying independent contractor marketers based on the number of tests referred or the dollar value of reimbursements,” Orkand adds.
Compliance tip: Be wary of a marketing company that recruits patients to a physician for the purpose of ordering routine labs tests, such as allergy testing, that may not even be medically necessary.
Telehealth relationships draw indictments
As part of a nationwide fraud takedown by the DOJ in late June, authorities charged 36 defendants, including labs, for allegedly submitting $1.1 billion in fraudulent reimbursement claims to Medicare resulting from telemedicine arrangements. The number of lab defendants was notable, Tangorre says.
For example, in New Jersey and Texas, “clinical laboratory owners allegedly paid illegal kickbacks and bribes, including to telemedicine companies, in exchange for the referral of orders for unnecessary genetic testing,” the DOJ stated.2 “The results of these genetic tests … were not used in the patients’ treatment.”
In an example from Houston, the owner of two labs is accused of working with another individual to obtain physician orders for genetic testing in exchange for kickbacks. Telemedicine doctors wrote up the orders even though they had no prior relationship with the patients they were serving, according to the DOJ.3
Compliance tip: Even labs that operate within the rules of the Medicare program should be on alert about possible telehealth fraud. Watch for situations in which the remote provider doesn’t appear to have enough contact with a patient to make a meaningful determination on whether tests are medically necessary.
Self-disclosure may appease authorities
Kingsbury points out that the government has reacted favorably to clinical labs that either came forth or offered up information voluntarily regarding possible Medicare fraud.
“A few 2024 cases stand out to us because they reflect a different priority: encouraging and rewarding self-disclosure and cooperation with government investigations by providing cooperation credit,” she explains.
For example, Guardant Health, a California-based laboratory, agreed in mid-July to settle allegations with the DOJ that it violated the False Claims Act and Stark Law by employing a referring physician’s family member. The physician ordered significantly more Guardant tests after the relative was hired, the settlement noted.4
Guardant eventually fired the family member after receiving information about the increase in test orders from the physician. The steps the company took after the revelation eventually led to “cooperation credit” and the settlement, suggesting a playbook of sorts that other labs should be aware of.
As highlighted by Kingsbury and Lovitch, those steps included the following:4
-
- Guardant made a submission under OIG’s self-disclosure protocol to alert the government of the problem.
-
- After learning about the potential violations, Guardant undertook an internal investigation and ceased all further billing for tests referred by the physician in question.
- The company cooperated with investigators by identifying individuals involved with the allegations; preserving and disclosing documents requested by authorities, disclosing facts gathered during its internal investigation, and assisting in the recovery of the losses to the Medicare program.
Guardant was not charged with any violations. It did, however, pay the government nearly $914,000 to settle the matter, and some of that money may have been paid in lieu of any formal charges.
“The settlement agreement indicates that $609,288.62 of this amount was restitution, suggesting that the government rewarded Guardant’s cooperation by applying a 1.5x multiplier to its damages calculation,” Lovitch explains. She adds that in such settlements, the multiplier is usually 2x, “but under the False Claims Act, damages of up to 3x are authorized by statute.”.
Honest labs still need to tread carefully
Observers should learn several important lessons from our special report:
-
- PGx and CGx testing represents a murky area that may hinge on a determination of medical necessity for the patients involved. Federal attention to this type of testing has only increased for all laboratories thanks to the actions of dishonest labs that sought illegal kickbacks or reimbursement for these tests.
-
- Labs that continue to use disreputable marketing firms without doing their homework risk getting caught in commission-based fraud enforcement.
- Telemedicine offers tremendous benefits to patients who are remote or otherwise unable to see physicians face-to-face. However, the underbelly of this technology is revealed when physicians, telemarketing platform companies, and laboratories take a short-sighted path in which doctors do not truly consult with patients prior to ordering diagnostic tests.
Labs that find themselves cornered by investigators—particularly if they violated healthcare fraud laws due to poor compliance practices rather than true criminal intentions—may do well to consider cooperating with authorities and taking their lumps in a settlement agreement rather than risking indictment from the DOJ.
References:
-
- Walters, Adam. G2 Intelligence. “Three Common PGx Compliance Traps for Laboratories to Avoid,” https://www.g2intelligence.com/three-common-pgx-compliance-traps-for-laboratories-to-avoid/
-
- U.S. Department of Justice, Office of Public Affairs. “National Health Care Fraud Enforcement Action Results in 193 Defendants Charged and Over $2.75 Billion in False Claims,” https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-193-defendants-charged-and-over-275-0
-
- U.S. Department of Justice, Criminal Division. 2024 National Health Care Fraud Enforcement Action, Case Summaries. https://www.justice.gov/criminal/criminal-fraud/health-care-fraud-unit/2024-national-hcf-case-summaries
- U.S. Department of Justice, Office of Inspector General of the Department of Health and Human Services. “Settlement Agreement.” Guardant Health settlement agreement with the DOJ: https://www.justice.gov/usao-ndca/media/1360656/dl?inline
__________________
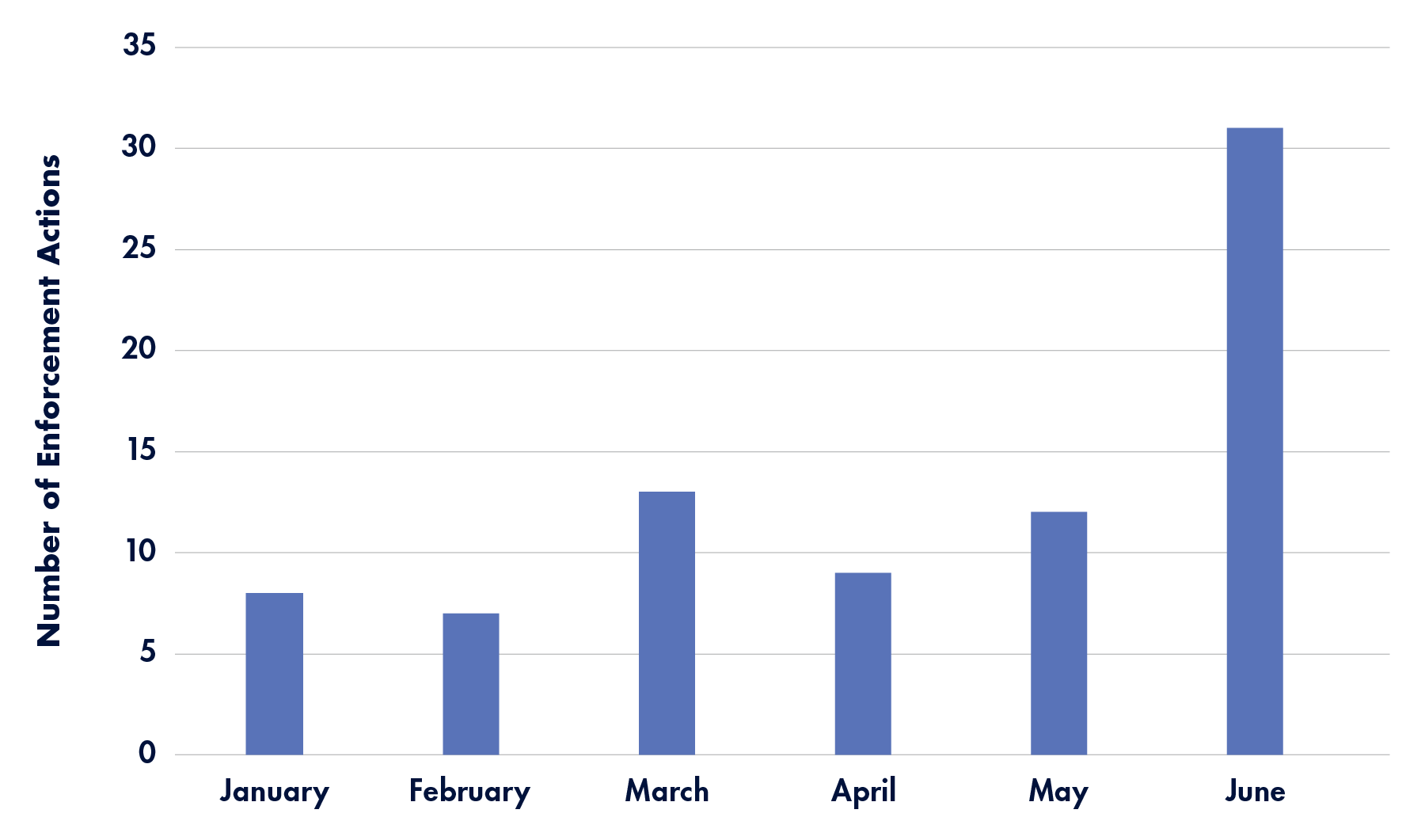
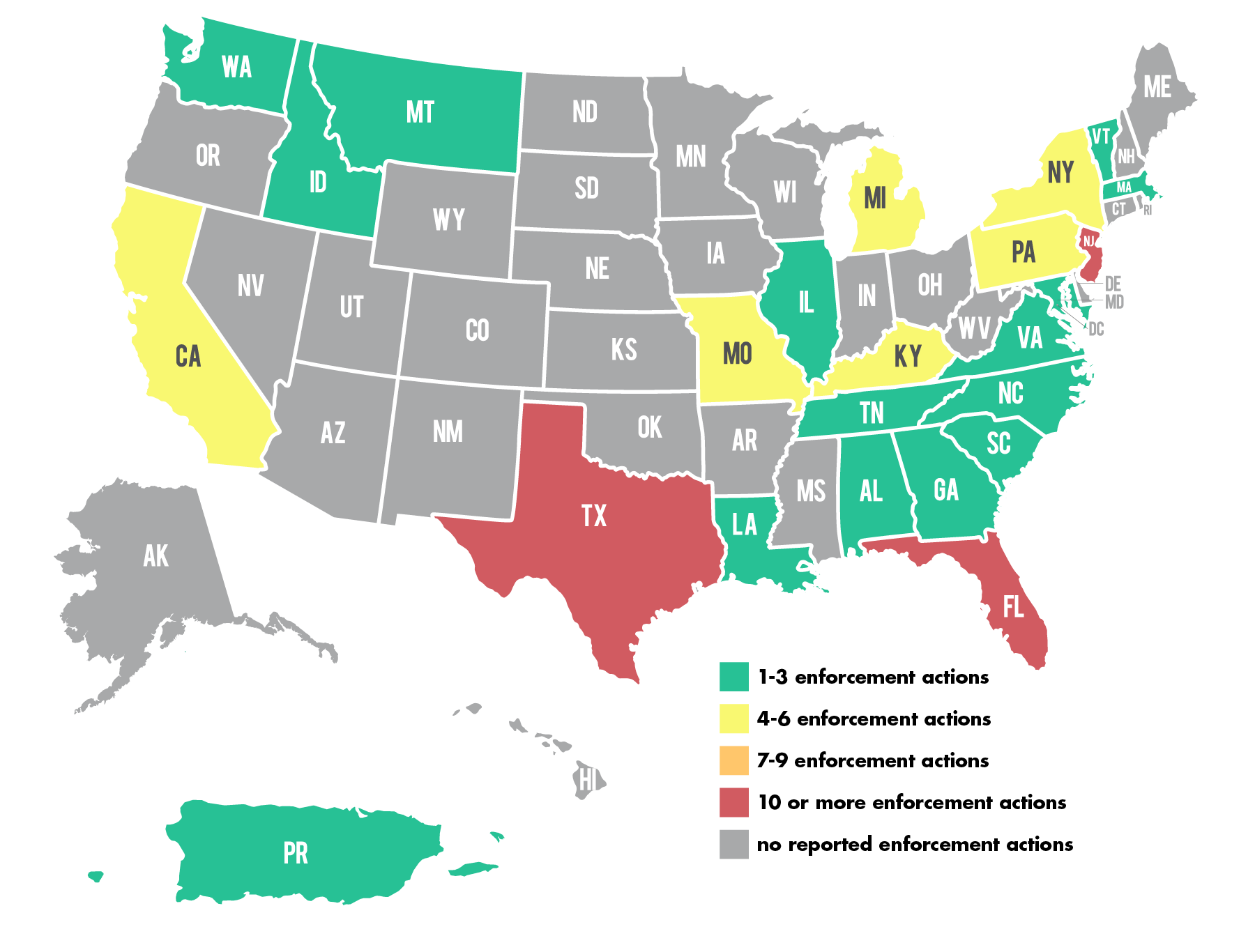
Timeline: 2024 Lab and Diagnostic Testing-Related Enforcement Actions
A month-by-month look at enforcement actions announced by the the U.S. Department of Health and Human Services Office of Inspector General—and related agencies—in the first half of 2024 that specifically mention laboratories or diagnostic testing:
January 2024
February 2024
The DOJ’s Civil Division released the latest statistics on False Claims Act settlements and judgments this month, which totaled $2.68 billion in fiscal year 2023.
March 2024
- Hollidaysburg Couple Pleads Guilty to Conspiracy to Defraud The United States And Husband Pleads Guilty To Health Care Fraud
April 2024
The DOJ’s COVID-19 Fraud Enforcement Task Force released its 2024 report this month, outlining COVID-19-related fraud and efforts to stop it. To date, the task force has charged 3,500 defendants, as well as seized or forfeited more than $1.4 billion in stolen COVID-19 relief funds.
May 2024
June 2024
- National Health Care Fraud Enforcement Action Results in 193 Defendants Charged and Over $2.75 Billion in False Claims (includes 21 lab/diagnostics-related actions from multiple states)
Subscribe to view Premium
Start a Free Trial for immediate access to this article