
Medicare Part B to Cover New CLIA-Waived Tests on Oct. 1
Make sure your lab billing staff is aware of the changes to ensure full reimbursement.

Make sure your lab billing staff is aware of the changes to ensure full reimbursement.

Based on validation study data, the test offers 90 percent accuracy and is unaffected by the emergence of new COVID-19 variants.

The new guidelines offer patients advice on when they should start their screening, and how often they should have it done.

For labs, the big takeaway is how prominently molecular testing and profiling figures in so many aspects of the NCCN’s recommendations.
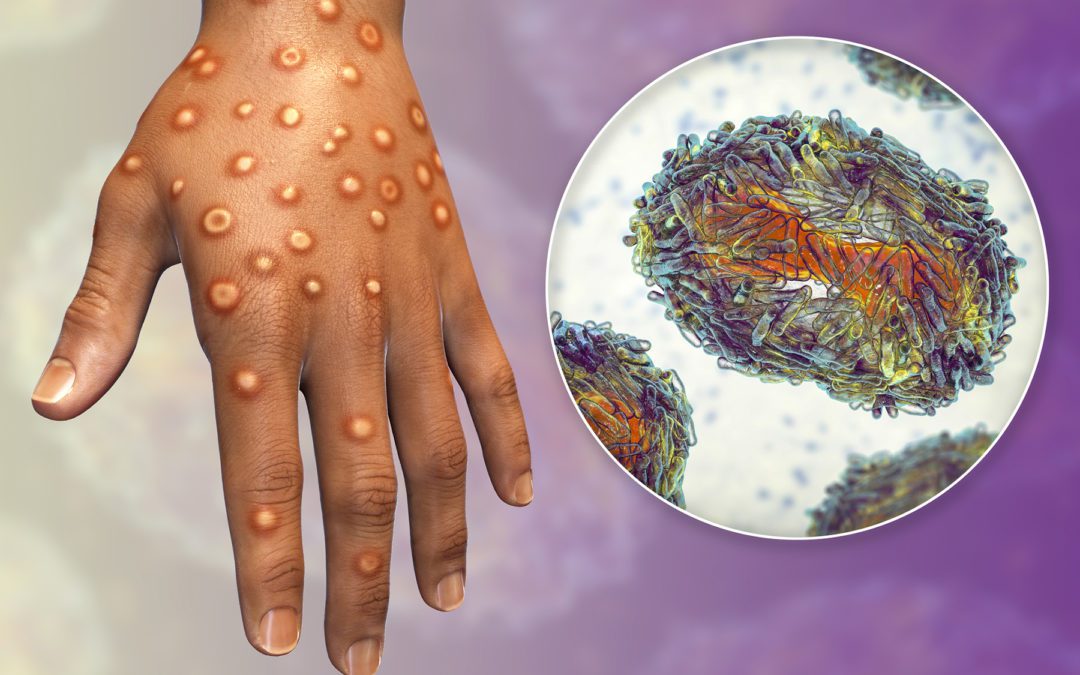
As the outbreak continues to grow in countries that have not historically seen monkeypox cases, testing companies are stepping up.