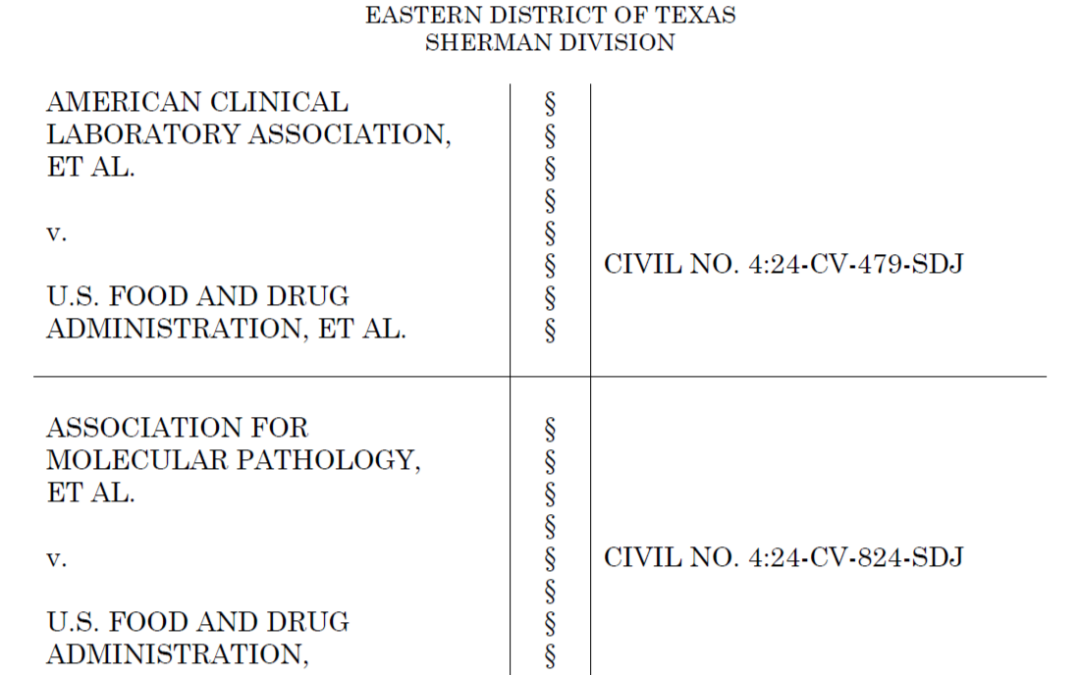
LDT Oversight Remains Under CLIA, for Now
Judge rules that the FDA has ‘no authority’ to issue its rule governing laboratory-developed tests

Judge rules that the FDA has ‘no authority’ to issue its rule governing laboratory-developed tests

Roche experts discuss the company’s recently launched automated solution and how it will bring mass spec to more patients

ACLA president Susan Van Meter discusses some of the key issues labs faced in 2024, along with work to address these developments through 2025

Regulatory expert Julie Ballard shares her thoughts on the most useful resources for labs as they get ready to comply with Stage 1

Experts say new leadership could impact the cost and availability of IVDs as well as patients’ ability to afford lab testing