
PAMA Alert: Hopes for SALSA Relief Die at the Eleventh Hour
Congress fails to include SALSA in the omnibus federal government spending bill passed at the end of 2022, but delays PAMA price cuts once again.

Congress fails to include SALSA in the omnibus federal government spending bill passed at the end of 2022, but delays PAMA price cuts once again.
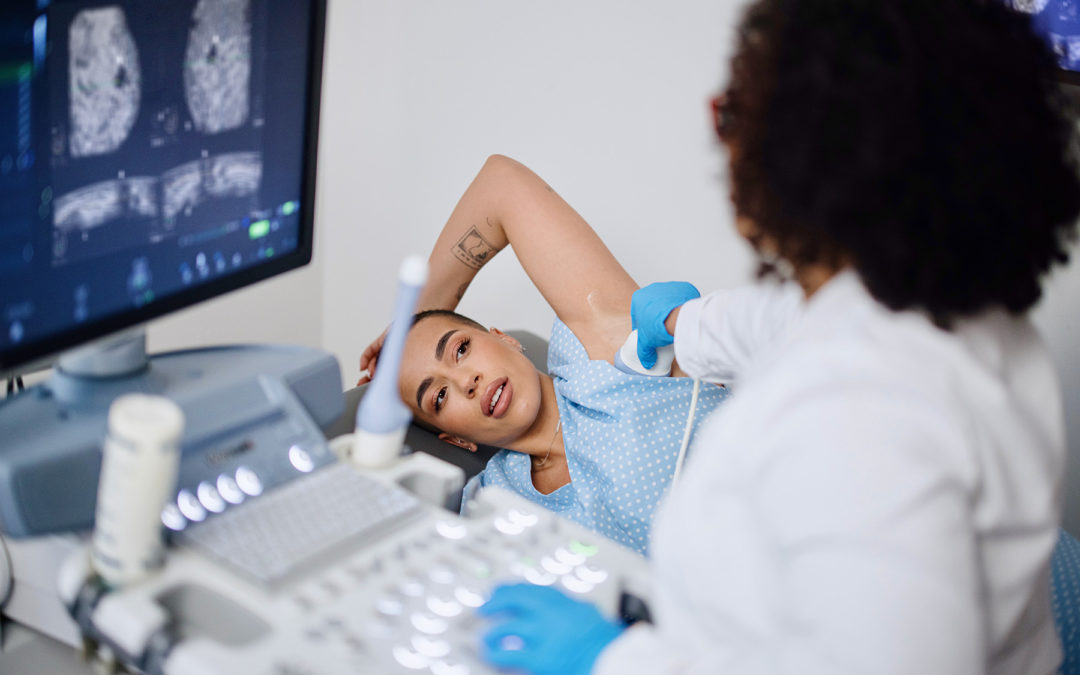
The FDA recently confirmed that a new rule requiring healthcare providers to notify patients of their breast density status will be released in late 2022 or early 2023.

FDA confirms the new rule, which will update the Mammography Quality Standards Act, will publish before the end of 2022, or early 2023.

With temporary waivers of restriction relating to telehealth likely to end soon, here’s a roundup of the current state of state telehealth licensing law.

For labs and other healthcare providers, a bill now before the Senate could mean a less labor-intensive prior authorization process.