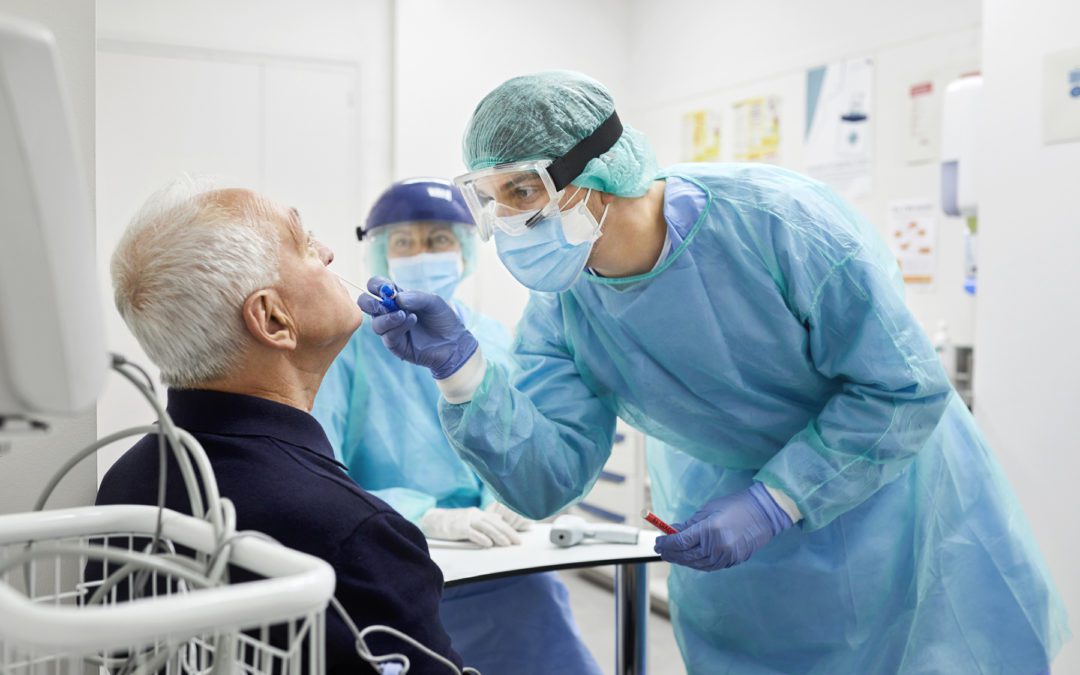
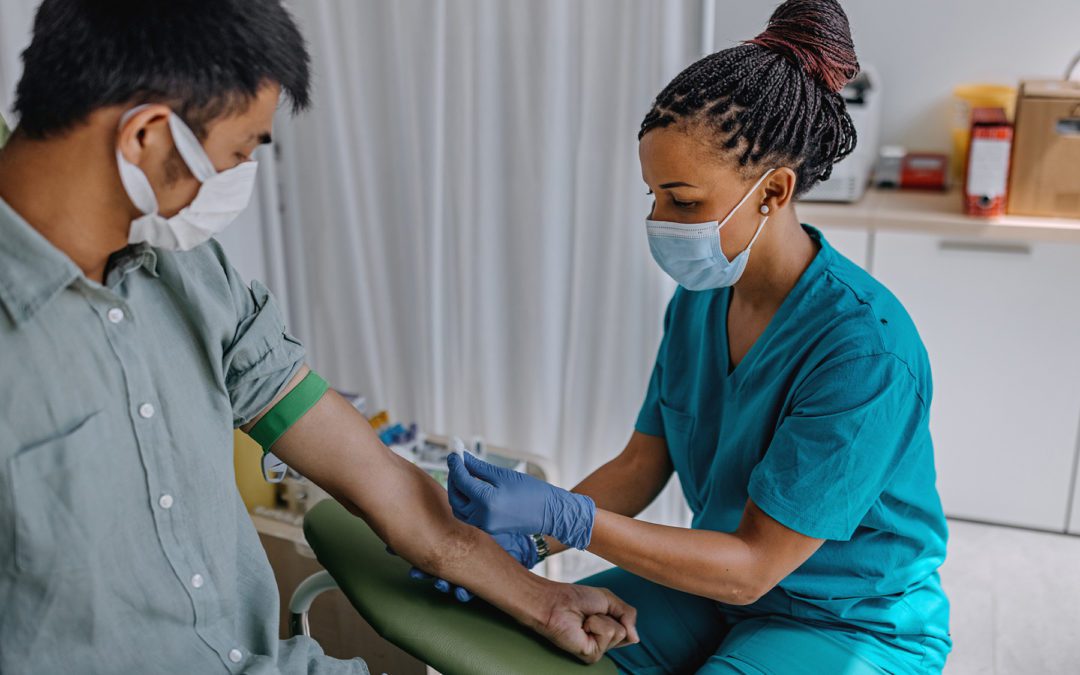
GAO: FDA Needs Policy to Avoid Repeating EUA COVID-19 Test Mistakes
In a recent report, the GAO says the agency needs a policy when it comes to enforcement discretion regarding tests approved via EUA.

In a recent report, the GAO says the agency needs a policy when it comes to enforcement discretion regarding tests approved via EUA.
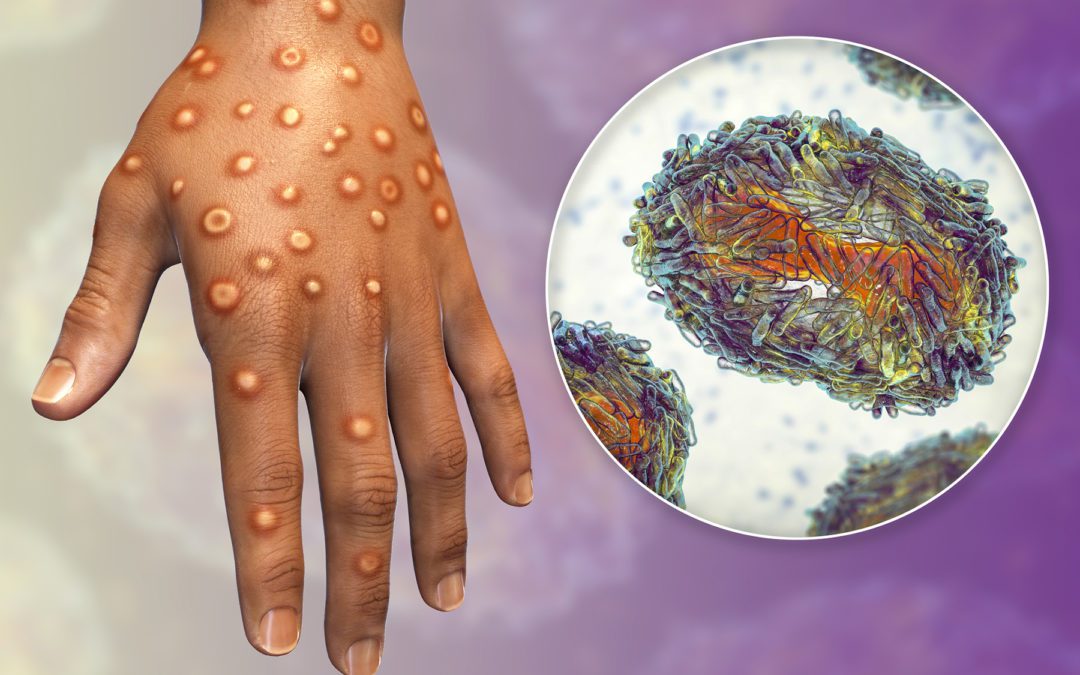
As the outbreak continues to grow in countries that have not historically seen monkeypox cases, testing companies are stepping up.
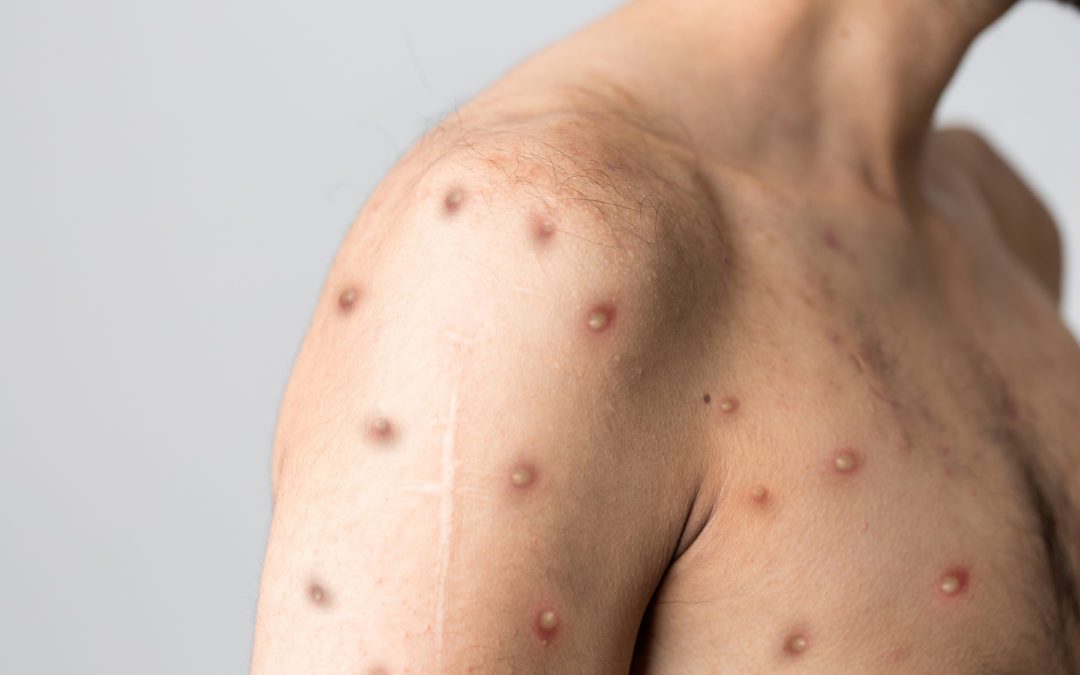
Recent FDA Safety Communication warns of the potential of false results from other sample types when testing for the monkeypox virus.
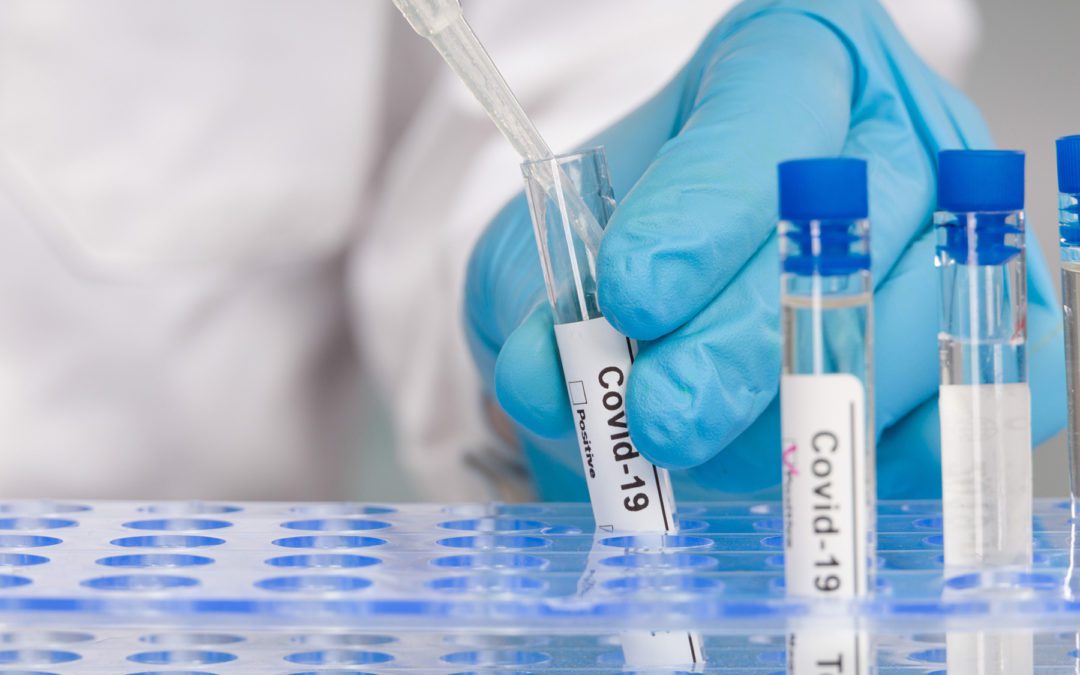
The agency needs a plan on use of EUA authority to get lab tests to market during the next public health emergency, says the GAO.
Recently released CDC data show that the largest drops occurred among groups who are most affected by HIV.