Expert Q&A: Quality, Proficiency, and CLIA Updates
Lighthouse Lab Services chief quality officer Anna Hill offers advice on keeping your lab compliant with the latest CLIA changes.

Lighthouse Lab Services chief quality officer Anna Hill offers advice on keeping your lab compliant with the latest CLIA changes.

The FDA is adding International Organization for Standardization (ISO) guidelines to its “good manufacturing” requirements
Individualized quality control plans offer flexibility and the capacity to seamlessly adopt new nonwaived tests, practices, and technologies.
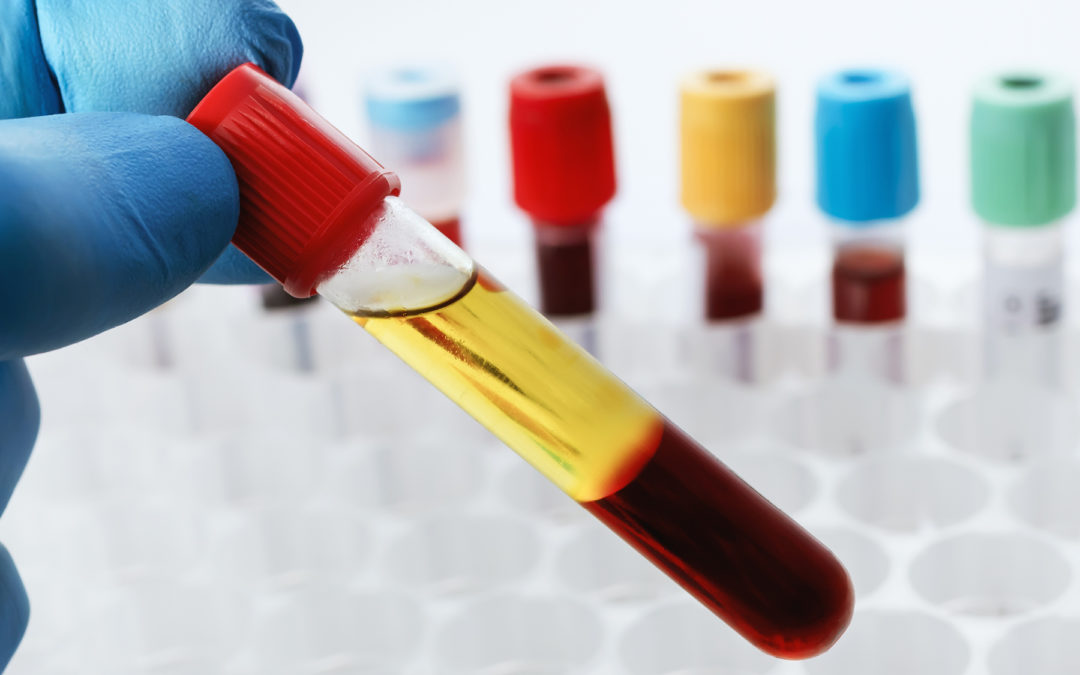
Experts share new strategies for dealing with hemolysis, icterus, and lipemia interference.
