
Privacy in the Post Roe v. Wade World: How Could Proposed HIPAA Restrictions on Reproductive PHI Affect Labs?
Key points labs should know about the recently proposed OCR rule on privacy protections for PHI associated with reproductive health care.

Key points labs should know about the recently proposed OCR rule on privacy protections for PHI associated with reproductive health care.

New ACMG recommendations aim to make CFTR carrier detection more equitable among a wider range of ancestries.

In a new practice guideline, the group also “strongly recommends” that NIPS be offered to patients to screen for fetal SCA.

The new guidelines address issues with false negatives and positives from POC testing for fertility and reproductive health.
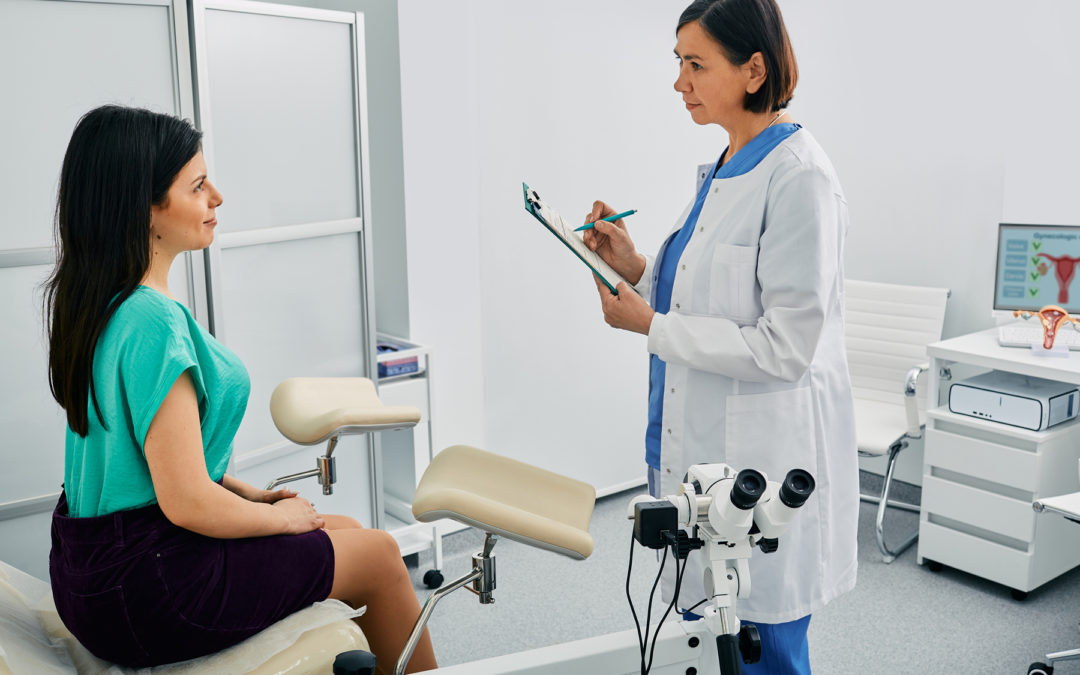
POC tests offer speed and simplicity, but are less reliable than tests performed in labs.